Updated/reviewed by the authors, July 2017.
Neovascular Phenotypes: Retinal Angiomatous Proliferation (RAP) or Type 3 Neovascularization
Authors:
Rufino Silva MD, PhD
João Pedro Marques, MD, MSc
Department of Ophthalmology, Centro Hospitalar e Universitário de Coimbra (CHUC), Coimbra, Portugal
Association for Innovation and Biomedical Research on Light and Image (AIBILI), Coimbra, Portugal
Faculty of Medicine, University of Coimbra (FMUC), Coimbra, Portugal
Introduction
Over 100 years ago, Oeller described for the first time the presence of anastomoses between the retinal and choroidal circulations in eyes with disciform scars(1).
Later, these were recognized in association with laser photocoagulation(2), radiotherapy(3), chorioretinal inflammatory diseases(4) and parafoveal telangiectasias(5).
The interest in this condition even led to anatomopathological studies in disciform scars from late age-related macular degeneration (AMD)(6).
In 1992, Hartnett et al.(7) described nine cases of retinal neovascularization, to which they referred as “deep retinal vascular anomalous complex”.
In 2000, Slakter et al.(8) described chorioretinal anastomosis in eyes with pigment epithelial detachment (PED) and indocyanine green angiography (ICG-A) hot spots but it was Yannuzzi et al.(9) who coined the term retinal angiomatous proliferation (RAP) when in 2001 the authors described chorioretinal anastomoses as a neovascular proliferation originating deeply in the retina.
In 2001, Yannuzzi et al. described chorioretinal anastomosis as neovascular proliferation with origin in the retina, and proposed the designation of RAP – retinal angiomatous proliferation(9).
Still, several authors(10-13) maintained the designation of chorioretinal anastomosis, proposing a choroidal origin for this clinical entity.
In 2008, Yannuzzi et al.(14) escribed 5 cases of RAP with the neovascular complex originating in the choroid instead of the retina, and proposed that RAP should be called type 3 neovascularization instead. The two terms have been used interchangeably ever since(15).
However, RAP is still the most common designation.
Classification
Based on the presumed origin and evolution of the neovascular process, a three-stage classification was proposed by Yannuzzi et al.(9), to characterize the clinical manifestations and progressive changes observed in RAP. However, it is often clinically difficult, if not impossible, to determine exactly when progression occurs from one stage to another.
Stage I – Intraretinal neovascularization.
The presence of small intraretinal hemorrhages is a hallmark of RAP and a very useful sign for its early clinical diagnosis. A small elevation of the inner/intermediate retina caused by angiomatous tissue may be observed under the slit lamp; this elevation may extend tangentially, assuming a telangiectatic appearance. Dilated retinal vessels may perfuse and drain the intraretinal neovascularization and form retino-retinal anastomoses (RRA)(9).
The fluorescein angiography (FA) of the intraretinal neovascular complex shows a focal hyperfluorescent area in front of the retinal pigment epithelium (RPE), mimicking classic choroidal or, more frequently, occult neovascularization. ICG-A may reveal a hot spot with staining and leakage.
Stage II – Subretinal Neovascularization
ovascular complex shows a focal hyperfluorescent area in front of the retinal pigment epithelium (RPE), mimicking classic choroidal or, more frequently, occult neovascularization. ICG-A may reveal a hot spot with staining and leakage.
Stage II – Subretinal Neovascularization
Involvement of the subretinal space with localized neurosensory retina detachment, edema and retinal hemorrhages at the edges may already be observed in colour fundus photography. Usually, a clear RRA can be seen, with a perfusing retina arteriole and draining venule communicating within the core of the subretinal neovascularization (Figure 1).
Stage III – Choroidal Neovascularization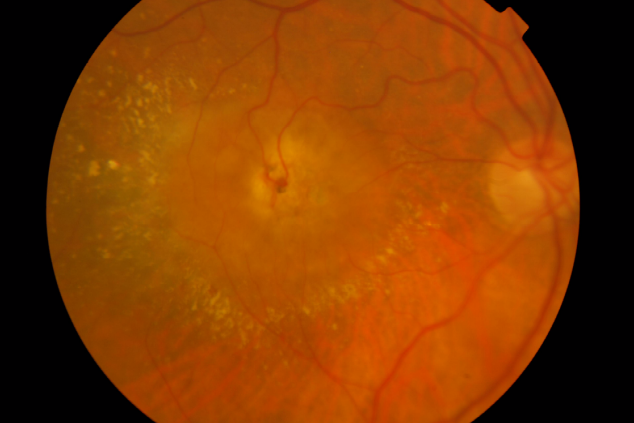
Figure 1. RAP lesion – Colour fundus photography of a RRA in a patient with RAP.
In stage II, an associated serous PED can be observed in 94% of the eyes(9).
FA may reveal a well-limited hyperfluorescence area enclosed in the diffuse leakage area associated with the PED, identical to that occurring on a minimally classic choroidal neovascular membrane. ICG-A clearly establishes stage II as the presence of a hot spot with leakage, as well as a hyperfluorescent area associated with serous PED.
Leakage may not be revealed by FA, probably because fibrin from retinal exudates cannot be impregnated with fluorescein, opposing to what occurs with ICG-A(16).
In fact, FA is rarely useful in the differential diagnosis between classic, occult or minimally classic membranes and early RAP lesions (stages I and II)(9).
On the other hand, ICG-A reveals a slow-growing extra, juxta or subfoveal hot spot, even in asymptomatic patients with RAP stages I and II.
Stage III – Choroidal Neovascularization
Stage III is established when the clinical and angiographic examinations can clearly demonstrate the presence of choroidal neovascularization (CNV), sometimes with the appearance of a vascularized PED.
According to Gass(11), RAP would progress in 5 stages instead of 3, easily identifiable by FA and ICG-A:
I) Pre-clinical stage - Atrophy of the outer retina, with retinal capillaries moving closer to a choroidal neovascular complex located below the RPE - type 1 CNV – and no clinical signs of chorioretinal anastomosis. ICG-A would be necessary to identify type 1 neovascularization.
II) Early clinical signs - Involvement of the subretinal space with localized neurosensory detachment of the retina, oedema and retinal haemorrhages at the edges may already be observed in fundus colour photography.
Anastomosis between dilated capillaries of the deep retina and the choroidal neovascular complex is associated with small intraretinal hemorrhages, which would constitute the first clinical sign of chorioretinal anastomosis.
This stage may occur weeks or months before stage III, where subretinal CNV is already observed.
III) Proliferation of CNV over the RPE - subretinal neovascularization – type 2 CNV.
IV) Appearance of serous PED - caused by activation of newly formed subepithelial vessels.
V) Mixed neovascularization - piggyback-type neovascularization, with two levels – type 1 and type 2 with cicatricial disciform lesion, making chorioretinal anastomosis visible.
Stage III in the Gass classification(11) corresponds to stage I in the Yannuzzi classification(9), with stages IV and V in the Gass classification(11) corresponding to stages II and III, respectively. Currently, the Yannuzzi classification(9) is the most widely used.
Recently, an optical coherence tomography (OCT) based classification was developed by Su et al.(17) According to the authors, a precursor stage consisting of punctate hyperreflective foci in the outer retina is the first OCT finding:
Stage 1 consists of a larger intraretinal hyperreflective lesion associated with cystoid macular edema but without outer retinal disruption.
Stage 2 is notable for outer retinal disruption that occurs with RPE disruption in most of the cases. Cystoid macular edema is generally present.
Stage 3 is defined by an intraretinal hyperreflective lesion that extends through the RPE to vascularize a drusenoid/serous PED. Cystoid macular edema is generally present.
Clinical Findings
The presence of small hemorrhages, soft drusen, and pigmentary changes is well documented in eyes with RAP and in unaffected fellow eyes, both in caucasian and in asian populations(9,18-21).
Marques et al.(19) conducted a quantitative evaluation of the fundoscopic features of fellow eyes of RAP and compared it with a cohort of fellow eyes of typical exudative AMD.
The authors reported that the total number and area of drusen are significantly smaller in fellow eyes of RAP than in fellow eyes of typical AMD.
Aside from soft drusen, a high frequency of reticular pseudodrusen in eyes with RAP has also been described(18).
The presence of telangiectasias, microaneurysms or punctiform retinal hemorrhages and RRA associated with intraretinal fluid is highly suggestive of RAP (Figure 2), as is the sudden disappearance of a retinal vessel that appears to have moved deeper(8-14,19,22).
Hard exudates usually accumulate around the central retinal neovascular complex, bordering the PED in stage III RAP lesions.
The advent of OCT, fundus autofluorescence (FAF) and infrared (IR) imaging allowed for a better characterization of the structural changes associated with the disease(23). Figures 2, 3, 4, 5 and 6 show clinical examples of patients with RAP who underwent multimodal retinal imaging.
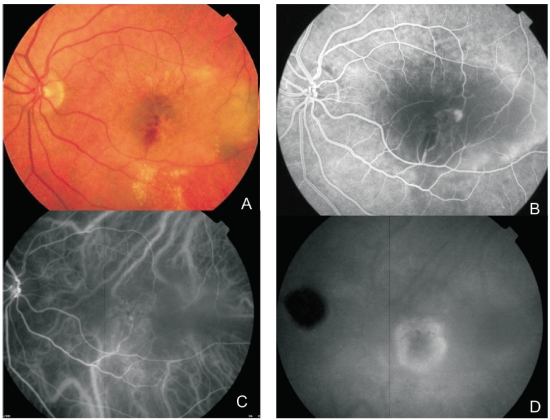
Figure 2. Multimodal retinal imaging of a RAP lesion. (A) Colour fundus photography displaying intraretinal hemorrhages, hard exudates and neurosensory retinal detachment. (B) FA shows a retinal hiperfluorescent spot apparently at the end of one retinal vessel, an intraretinal hemorrhage, neurosensory retinal detachment and PED. (C) Early phase ICG-A with an intraretinal hot spot (angiomatous proliferation) over diffuse choroidal hyperfluorescence. (D) Late phase ICG-A reveals a subfoveal hiperfluorescent plaque (subretinal neovascularization).
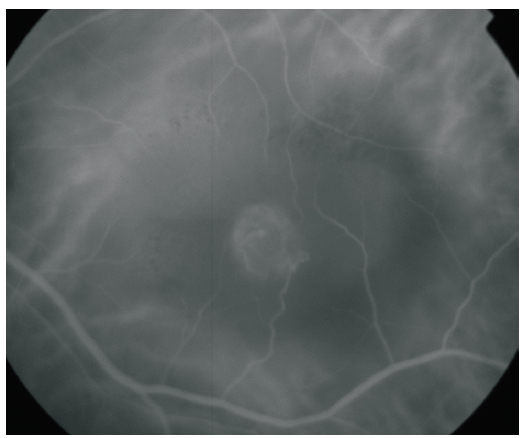
Figure 3. ICG-A of a RAP lesion. A RAP lesion with RRA, an apparently intraretinal angiomatous mass and a serous PED, imaged with ICG-A.
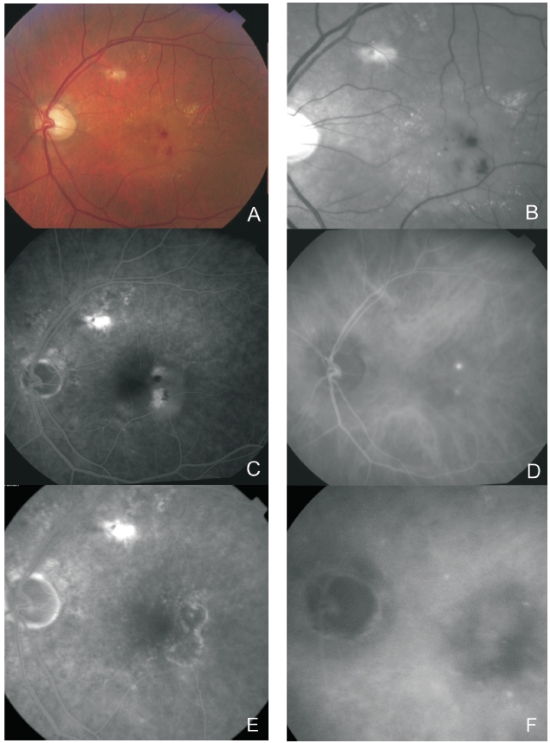
Figure 4. Multimodal retinal imaging of a RAP lesion. Colour fundus photography (A) with intraretinal hemorrhages, hard exudates and neurosensory detachment. Red-free imaging (B) with two juxtafoveal and extrafoveal small hemorrhages. FA (C) shows two juxtafoveal hyperfluorescent spots, neurosensory detachment and PED. Late ICG-A (D) reveals two juxtafoveal hyperfluorescent hot spots. FA (E) and ICG-A (F) after treatment with laser photocoagulation showing resolution of the exudation.
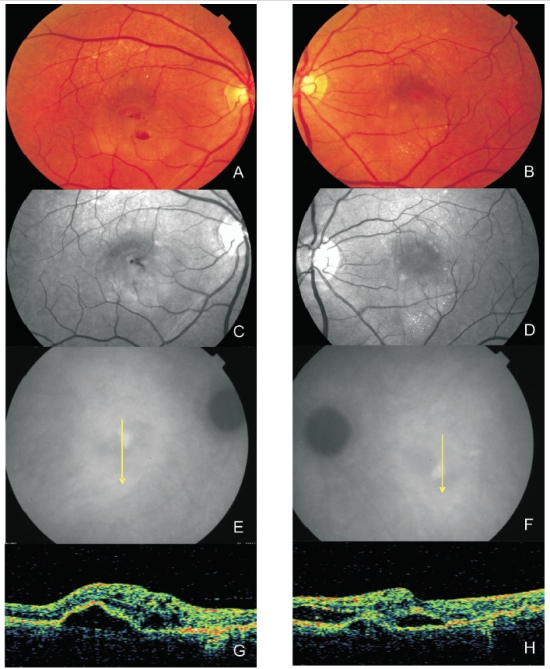
Figure 5. Bilateral RAP lesion: Colour fundus photography (A and B) and red-free images (C and D) clearly show small retinal hemorrhages and lipid exudation. (E and F) Late ICG-A of both eyes with a hot spot and subfoveal hypofluorescence (serous PED). Stratus OCT (G and H) reveals serous PED, neurosensory retinal detachment and intraretinal fluid in both eyes.
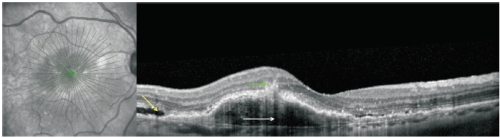
Figure 6. RAP lesion imaged with spectral-domain (SD)-OCT. SD-OCT of a RAP lesion with an area of intraretinal neovascularization (green arrow) in the deep retina with a RPE detachment (white arrow). Intact and ruptured portions of the Bruch’s membrane and subretinal fluid (yellow arrow) can also be seen.
The recent introduction of OCT angiography (OCTA) in clinical practice allowed for an unmet morphological characterization of RAP lesions.
Qualitative and quantitative analyses of type 3 neovascular complexes can be performed using OCTA(24,25).
Querques et al.(26) evaluated the features of RAP with this new imaging modality and found lesions emerging from the deep capillary plexus, forming a clear, tuft-shaped, high-flow network in the outer retinal segment in all eyes, abutting in the sub-RPE space.
The authors also reported a small, clew-like lesion present in the choriocapillaris and that, in some cases, this clew-like lesion seemed to be connected to the choroid through a small-caliber vessel.
Compared with conventional imaging, OCTA may improve the detection and delineation of vascular changes occurring in RAP(25).
Further longitudinal studies with OCTA may bring further insights on the pathophysiology of RAP and establish OCTA imaging as an additional biomarker to guide the treatment and monitoring of these lesions.
Differential diagnosis
Differential diagnosis is mandatory for:
- Other forms of CNV, namely occult CNV
- Polypoidal choroidal vasculopathy (PCV)
- Macular telangiectasia (MacTel)
Other forms of CNV with ICG hot spots (occult CNV) and PCV are two important diagnoses that can be easily confounded with RAP.
Small intraretinal hemorrhages, sometimes punctiform, in patients with soft drusen, are very typical in RAP, as are telangiectasias and RRA.
On the contrary, retinal hemorrhages in PCV are normally larger, with round reddish-orange nodules usually being observed in the fundus(27).
OCT is a useful tool in the differential diagnosis between RAP, PCV and occult CNV(28,29).
In RAP, intraretinal hyperreflectivity may be observed, corresponding to angiomatous proliferation associated with intraretinal fluid and/or RPE detachment(30,31).
In PCV, polyps appear in OCT as abrupt protrusions from the RPE/Bruch’s membrane band, often associated with neurosensory detachment(27,28).
The double layer sign, RPE irregularity and a notched PED are hallmarks of PCV on OCT(32).
MacTel is a condition involving dilation of retinal capillaries located near the fovea, in one or both eyes.
RPE hyperplasia may also occur, with refractive punctiform deposits and macular leakage being observed in FA.
Migration of one or more venules to the deep retina can also be found(5).
Also, anastomoses between retinal vessels and the choroidal circulation have been described, as well as choroidal new vessels.
The most significant differences are the fact that MacTel are not associated with serous PED, the RPE is healthier and CNV occurs less frequently(5,22).
Natural history
Natural history may be highly variable and in many aspects similar to that of other neovascular AMD lesions.
RAP has been coined a bilaterally aggressive disease with predictable symmetry(9,33,34).
In fact, the vasogenic potential associated with RAP has been highlighted by several group(18,33,35), reporting annual and cumulative rates of neovascularization in fellow eyes far exceeding the typical forms of exudative AMD.
Yannuzzi et al.(9) reported an average of 15 months until the development of neovascularization in their cohort of fellow eyes of RAP.
In a cohort of 52 fellow eyes of RAP, Gross et al.(34) reported a cumulative incidence of neovascularization of 40% at 12 months, 56% at 24 months and 100% at 36 months.
Campa et al.(33) have also evaluated the cumulative incidence of neovascularization in a population of 37 fellow eyes of RAP but found a significantly inferior conversion rate (36.4% at 36 months).
Recently, a small cohort (20 fellow eyes of RAP) in a Japanese population(18) identified an incidence of neovascularization of 50% at ~49 months of follow-up (min 24; max 108 months).
RAP was historically associated with a poor natural history and high relapse rates. Kuhn et al.(10) studied 22 eyes and observed structural evolution towards classic membranes, signs of RPE rupture and fibrous scars in 36.4%, 4.6% and 31.8% of cases, respectively. The authors described a decrease in visual acuity (VA) in 77% of cases. Similarly, Hartnett et al.(36) reported that 9/11 patients they evaluated developed legal blindness.
A study by Viola et al.(37), where the authors investigated the natural history and visual outcome in 14 eyes with untreated RAP for an average of 20 months, found that, by the time of the final examination, VA had decreased to ≤20/200 in 11 eyes (69%), and 5/14 patients (36%) were legally blind. However, while several treatment approaches proved unsuccessful in the past(13,38), the blockade of vascular endothelium growth factor (VEGF) has considerably changed the paradigm of this condition, with recent studies showing similar functional results to typical exudative AMD(39,40).
An important feature of the natural history of RAP lesions is geographic atrophy (GA).
It was previously thought that GA in RAP was secondary to the treatments used(41,42).
However, in a recent study using FAF, McBain et al.(43) found high rates of GA in RAP, independently of treatment modalities (phtotodynamic therapy or anti-VEGFs) and other authors have reported similar results(39,44).
This finding may be a consequence of the reduced choroidal perfusion and reduced choroidal thickness in RAP, which can be the reason behind the high vasogenic potential reported with this disease(21,43,45).
Epidemiology
RAP is now considered a well-established neovascular phenotype of AMD, with distinct clinical and epidemiologic features, most likely due to genetic differences still not completely understood(46) .
Environmental risk factors such as age and arterial hypertension have been associated with the development of both RAP and typical exudative AMD(47) .
Even though the role of arterial hypertension is still matter of debate, it is now widely accepted that RAP patients are significantly older than typical exudative AMD patients – average age of 79 vs. 76 years(6,8,9,14,47).
The average and median ages of a series of 108 patients studied by Yannuzzi et al.(9) were 80 and 81 years, respectively.
Women are more frequently diagnosed with RAP, comprising 64.7% to 71% of the affected individuals(9-10,47).
In caucasian populations, RAP is estimated to represent 5-28% of wet AMD cases(9,15,34,40,48,49).
Using the ImageNet 1024 videoangiography system, our team observed a prevalence of 9.4% in a consecutive series of 563 patients(13).
Studies in asians suggest a considerably lower prevalence(35,50) and there are no known reports of RAP in blacks(14).
Prevalence appears to be greater in hyperpigmented eyes(8,9,51).
Rapid progress in identifying genetic risk factors for AMD susceptibility has been made over the last few years, including the identification of two major loci at chromosome 1q32 and 10q26(46,52).
Even though several studies have evaluated genetic risk factors for exudative AMD, very few have specifically addressed the genetic alterations found in RAP(46,53,54).
Wegscheider et al.(53) and Caramoy et al.(47) suggest that CFH gene variants are less frequent in patients with RAP than in other forms of exudative AMD, while ARMS2 variants, namely the single nucleotide polymorphism (SNP) A69S (rs10499024), appears to have a stronger association with this neovascular phenotype(46,54,55).
These findings stress the importance of detailed phenotyping in AMD in order to identify genetic biomarkers for the distinct AMD subtypes.
Anatomopathology
In 2000, Lafaut et al.(16) studied six “deep retinal vascular anomalous complex” lesion specimens from retinal translocation surgery in eyes evaluated with FA and ICG-A.
The deep vascular anomalous complex was located in front of the RPE, thus not representing CNV. Nodular fibrovascular complexes surrounded by a ring of diffuse drusen – basal linear deposits under electron microscopy –, pigment epithelium and an amorphous fibrous material containing photoreceptor outer segment debris were observed.
Even though the lesion was not covered by RPE, the latter was preserved around the lesion. The amorphous material did not cover the retinal surface of the membrane, which adhered directly to the outer nuclear layer in 50% of cases.
Anastomoses between fibrovascular nodules and the choroidal circulation were only observed in cases showing isolated disciform scars.
Avascular fibrocellular membranes were observed on the inside of Bruch’s membrane and, in three cases, on the choroidal side of diffuse drusen.
Fibrin was present in affected retinas; choroidal anastomoses were not observed. Still, the presence of retino-choroidal anastomoses could not be entirely excluded, since the specimens may not have included the entire lesion.
In a histopathological study performed in nine neovascular lesions classified as RAP, Shimada et al.(56)
found only intraretinal neovascularization in stage II cases.
In stage III cases, these authors found choroidal and intraretinal neovascularization; they concluded that their findings were in agreement with the classification proposed by Yannuzzi et al.(29).
Furthermore, the authors identified the expression of VEGF (in intraretinal neovascularization and in the RPE), CD68-positive macrophages (in the neovascularization area) and expression of hypoxia-inducing factors (HIF) alpha 1 and alpha 2 in neovascular endothelial cells.
They hypothesized that intraretinal neovascularization would appear before the occurrence of CNV and be associated with ischemia and increased expression of VEGF and inflammatory factors.
Monson et al.(57)described the histopathological characteristics of RAP in an 87-year-old woman.
The images obtained by fundus examination and FA were histopathologically consistent with a neovascular intraretinal angiomatous complex without subretinal pigment epithelial neovascularization.
Gass et al.(11) described chorioretinal anastomoses and atrophy of the outer nuclear layer in a pre-clinical case, with outer retinal capillaries moving close to a CNV focus, having proposed a choroidal origin for RAP, instead of the retinal origin initially proposed by Yannuzzi et al.(9).
The origin of the neovascularization process (choroidal or retinal) in RAP remains a controversial issue.
Regardless, the anatomopathological studies helped elucidate that RAP lesions are associated with ischemia, age-related macular alterations, increased VEGF production and macrophage expression, similar to other forms of AMD-related CNV.
Pathogenesis
Kuhn et al.(12)studied the evolution of RAP in the second eye of two patients.
The authors assumed the existence of two asymmetrical membranes, a smaller retinal membrane – the angiomatous anomaly – over a larger membrane – the choroidal membrane, secondary to failure of a diseased RPE, unable to modulate and inhibit neovascular factors.
Even though the exact mechanism and origin of RAP remains elusive, the important role of VEGF cannot be overemphasized. The presence of serous PED may increase hypoxia by moving the retina even further from the choroid(36).
RPE decompensation is probably behind the deposition of amorphous materials in the subretinal space (under the basal lamina) and consequent thickening of Bruch’s membrane, all contributing to a decrease in inner retinal oxygenation.
Choriocapillaris atrophy and hypoxia may lead to intense neovascular activity, with reactive synthesis of growth factors, such as VEGF(58).
Overexpression of VEGF is sufficient to produce intraretinal neovascularization and subretinal neovascular membranes in animal and human models(58,59).
The relatively good response of these lesions to ranibizumab, bevacizumab and aflibercept confirms the central role of VEGF in the pathogenesis of RAP lesions.
As described above, Yannuzzi et al.(9) initially hypothesized that the initial neovascular process in RAP occurs in the deep retina and consists of 3 stages. Gass(11) contested a retinal origin for anastomosis, based on the following facts:
- no communication between retinal vessels and the choroid could be found in surgical specimens;
- the location of vascular anomalous complexes on the outer nuclear layer of atrophic retinas is more compatible with a choroidal origin hypothesis;
- although the macular retina is not excised in surgery, the retinal neovascular complex disappears and no macular hole is left, which contradicts its retinal origin.
It is clear that no definitive sequential histopathological evidence exists to support an intraretinal versus a choroidal origin for RAP lesions.
In 2008, Yannuzzi et al.(14) re-evaluated the early stages of RAP lesions in five eyes, using FA, ICG-A, time-domain-OCT and frequency-domain -OCT, having concluded that the initial lesion may have its origin not only on the deep retinal capillaries but also in the choroid.
They described RAP as “type 3 neovascularization”, a type of neovascularization with preference for the retina, displaying the following manifestations:
- Focal neovascular proliferation from the deep retinal layer (originally RAP);
- Intraretinal neovascular extension from underlying occult type I CNV (originally occult chorioretinal anastomosis);
- De novo breaks in Bruch’s membrane with neovascular infiltration into the retina.
The term type 3 neovascularization helps to resolve the various conflicting theories and descriptions behind the origin of RAP lesions: neovascularization in RAP may originate not only from the deep retinal capillaries but also from the choroid.
However, the main issue is not the intraretinal or choroidal origin of neovascularization but its unique characteristic of presenting two neovascular foci: one located in the deep retina and the other at a choroidal level.
Treatment
Before the anti-VEGF era, several treatment methods that included direct laser photocoagulation of the vascular lesion, laser photocoagulation of the feeder retinal arteriole, surgical ablation, scatter grid-like laser photocoagulation, photodynamic therapy (PDT), transpupillary thermotherapy, intravitreal triamcinolone acetonide (IVTA) and combined regimens were used, yielding at most VA stabilization, marginally better VA or short-term VA improvements(12,13,60,61).
Thermal laser Photocoagulation
Kuhn et al.(10) photocoagulated hot spots in 28 eyes, reporting “occlusion” in 25% of cases, including one eye with recurrence.
No occlusion was observed in the remaining 75% of cases, despite multiple treatment sessions.
Treatment caused tearing of the RPE in 45% of cases and VA decreased in 86% of eyes.
The low success rate might have been related to inadequate laser penetration – given the serous PED height or absorption of radiation by the sub-RPE fluid –, incorrect location or the presence of multiple afferent vessels.
Slakter et al.(8) confirmed this poor prognosis, particularly in serous PED cases. Occlusion was not possible in 86% of eyes and progressive evolution to a disciform scar was noticed.
Clinical experience showed that some extrafoveal stage I lesions (according to the Yannuzzi classification(9)) are amenable to laser photocoagulation(62).
In a series of 108 eyes with RAP, Bottoni et al.(60) achieved full obliteration of chorioretinal anastomosis in 57.1% of these lesions.
However, the risk of complications needs to be evaluated and careful follow-up is mandatory, given the high rates of persistence and recurrence.
Surgical ablation
Borrillo et al.(63) performed vitrectomy with detachment of the posterior hyaloid face, surgical section of afferent arterioles and draining veins and membrane excision in 4 eyes with RAP and PED – stage II – with resolution of intraretinal edema, collapse of the PED after 7-10 days and an increase in the average VA (20/200 pre-operative vs. 20/70).
Shimada et al.(56) excised the neovascular complex in 9 eyes from 8 patients – stages II and III.
VA remained stable in the post-operative period; however, significant destruction of the RPE and the choriocapillaris occurred in patients with serous PED.
The authors concluded that surgery may stabilize VA in stage III but is not indicated in stage II.
Surgical section of the afferent vessel associated with intravitreal injection of triamcinolone was performed in one eye, with resolution of exudation and VA of 20/320 at 6 months(64).
Nakata et al.(65) found surgical ablation (even combined with PDT) not useful for the treatment of RAP lesions, given the high frequency of reperfusion from retinal inflow vessels associated with this procedure.
Shiragami et al.(66) observed recurrence of RAP lesions in all 7 cases treated, 2 to 13 months after surgical ablation.
Surgical ablation of RAP is no longer recommended for RAP lesions, considering the better outcomes achieved with other treatment modalities, such as antiangiogenic drugs.
Photodynamic therapy (PDT) alone or in combination with intravitreal triamcinolone acetonide (IVTA)
Studies of PDT in monkeys with neovascular complexes and chorioretinal anastomoses, showed occlusion of the neovascular complex but not of the anastomoses(67).
The authors hypothesized that the anastomoses would be responsible for repermeabilization of the neovascular complex.
Kusserow et al.(68) treated 6 eyes with predominantly classic membranes and chorioretinal anastomoses without success.
No improvements in VA were observed for any of the treated eyes and membranes continued to grow.
Our group reported stabilization or improvement in VA after PDT in 73.3% of eyes (<3 lines loss) at 12 months(13), thus representing a better outcome than the natural evolution(36).
However, a significant VA decline was observed in the second year, mainly due to recurrence(12).
Boscia et al.(69) treated 13 eyes with PDT, having concluded that PDT would only be useful in cases where serous PED represents less than 50% of the lesion.
In 2006, these authors referred that early treatment of eyes with smaller lesions using PDT with verteporfin potentially led to a beneficial effect on vision, whereas it might worsen the natural progression of larger lesions, with most eyes undergoing enlargement, disciform transformation or RPE tear(70).
Reported short-term results of non-randomized studies on RAP lesions treated with PDT and IVTA(41,71,72) revealed apparently better VA outcomes and/or a reduced number of treatment sessions compared to PDT alone.
However, recurrences were also frequent(42,61).
Krebs et al.(73) found no significant differences between the PDT monotherapy group and the combined PDT and IVTA group regarding evolution of distance VA, retinal thickness and lesion size, having concluded that new therapeutic strategies might be required in RAP lesions, probably including therapy with antiangiogenic agents.
Antiangiogenic agents
Better visual outcomes can be achieved by treating RAP lesions with intravitreal ranibizumab(39,40,44,74,75), bevacizumab(76,77) and most recently aflibercept(78).
Data from clinical trials suggests that the response of RAP lesions to CNV treatments may be similar to that of other variants of neovascular AMD(79).
Improved VA and short-term edema reduction or elimination, were also observed in combined treatments using PDT and ranibizumab(44,80) or bevacizumab(81.82).
However, no superiority of combined treatment with PDT and ranibizumab or bevacizumab has been demonstrated.
The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) study followed up a large cohort of patients with treatment-naïve exudative AMD eyes, who received randomly assigned ranibizumab or bevacizumab for 2 years. The cohort included eyes with classic and occult CNV as well as RAP.
The authors recently published a comparison between eyes with and without RAP regarding the 2-year visual and morphologic outcomes(39).
This study showed a rapid improvement in VA in eyes with RAP within the first 3 months of intravitreal anti-VEGF therapy that continued to improve and then stabilized during the first year.
However, in the second year, VA began to decline modestly such that there was no statistically significant difference between eyes with or without RAP in overall VA gain at the end of the second year.
The authors noticed that fewer injections were needed to treat RAP than the other types of CNV but the rate of GA was higher in the RAP group(39).
A recently published study(44) on the long-term results (≥36 months of follow up) of PRN intravitreal ranibizumab for the treatment of RAP lesions in clinical practice (n=79), showed that ∼40% of the eyes had stable or improved VA at the end of follow-up, and about one fifth of the individuals preserved reading vision.
Nevertheless, about 60% of the patients presented with some degree of GA at the last visit.
The presence of subretinal fluid at baseline was found to positively correlate with a favorable visual outcome, thus appearing to be an important prognostic factor for functional improvement.