Modifiable risk factors for AMD
Author:
Cécile Delcourt, MD, PhD
Inserm, U897, Bordeaux, France Université Bordeaux 2, Bordeaux, France
1. Introduction
The epidemiological studies conducted in the past 25 years have helped identifying major modifiable risk factors for AMD. In particular, smoking and nutrition appear ever more important in determining the occurrence of AMD, and may, in the future, lead to prevention strategies.
2. Smoking
Smoking is the best characterized risk factor for AMD(1). The initial observations performed in Caucasian populations from Western countries(2), are now being confirmed in other ethnic groups, such as African-Americans(3), Latino-American(4), or Asian populations(5-7). In most studies, the risk for late AMD was multiplied by 2.5 to 4.5 in current smokers. In addition, the dose-response relationship was explored in some studies(6,8-12). Most of these studies found that the risk for AMD increased with increasing number of cigarettes smoked per day, and, even more, with number of pack-years smoked, which is an indicator of cumulative smoking over the lifetime (mean number of packs smoked/day x duration of smoking (years). Moreover, the risk for AMD appeared to decrease with time from cessation of smoking. Former smokers generally demonstrated a lower risk for AMD than current smokers. Several studies have shown that the risk for AMD in subjects having ceased smoking for more than 20 years was similar to the risk in never smokers(8-10,13). One study suggested that passive smoking is also associated with an increased risk for AMD(9), while this association did not reach statistical significance in another study(14). Finally, smoking appeared to be related to similar risks for both types of late AMD (geographic atrophy and neovascular AMD)(9,13,15-16). By contrast, associations with early AMD were weaker in the vast majority of published studies, and often not statistically significant(8-9,13,15,17-18).
Overall, the strength of the association (about 3-fold increased risk in current smokers), its consistency across different populations, the observation of a clear dose-response relationship in most studies, and the decrease of the risk with stopping smoking are all strong arguments in favour of a causal role of tobacco smoking in the aetiology of late AMD.
The exact mechanisms by which smoking increases the risk for AMD are unclear, and probably multiple, including oxidative stress, inflammation and decreased macular pigment. Finally, recent studies gave important insights on the joint effects of smoking and genetic polymorphisms, showing that the risk for AMD is particularly high in smokers bearing at-risk polymorphisms in the CFH or LOC387715 genes(19-21).
Other vascular risk factors, such as systemic hypertension, obesity, diabetes, plasma lipids or alcohol drinking may be associated with an increased risk of AMD, but epidemiological studies have been inconsistent in this field(22). At the time being, they remain putative, but not clearly identified risk factors for AMD.
Nutritional factors
More recently, epidemiological studies have focused on the potential association of AMD with nutritional factors. Mainly three types of nutritional factors have been investigated for their potential protection against eye ageing: antioxidants (mainly vitamins C and E, zinc), the carotenoids lutein and zeaxanthin and omega 3 polyunsaturated fatty acids (PUFA).
The retina is particularly susceptible to oxidative stress because of the high level of in-site reactive oxygen species production, due in particular to light exposure and high metabolic activity(23).
Epidemiological studies are mostly in favour of a protective role of antioxidants for AMD(24).
Moreover, the Age-Related Eye Diseases Study (AREDS), a randomized clinical trial performed in the United States and including on almost 5000 subjects supplemented for five years, showed a significant 25% reduction of the incidence of late AMD with supplementation in antioxidants and zinc, by comparison with placebo(25). In this field, data from the United States should be extrapolated to European populations with caution. Indeed, vitamin supplements are widely used in the American population, while this is rarely the case in Europe. For instance, two thirds of the AREDS participants used vitamin supplements, in addition to the supplementation tested in the study(25). Plasma vitamin C concentration at baseline in the AREDS (before the initiation of the study supplementation) was 62 micromol/l(25), whereas it was 31.6 micromol/l in men and 40.5 micromol/l in women of the Pathologies Oculaires Liées à l’Age (POLA) Study, performed in the South of France(26). Similarly, in the EUREYE Study, plasma vitamin C concentrations ranged from 35.5 micromol/l to 48.4 micromol/l in seven European countries(27). Therefore, antioxidant intake is much lower in European populations than in the United States, with part of European populations being at risk of clinical deficiency in these vitamins. Two European studies suggested that the benefit to be expected from increased antioxidant intake may be more important in our populations with low antioxidant intake. Indeed, in the French POLA Study, we observed an 80% decreased risk for late AMD in the subjects with higher plasma vitamin E, by comparison to those with lower concentrations(28), a much stronger effect than the 25% reduction in risk observed in the AREDS Study. Moreover, we observed a 25% reduction in risk for early ARM, whereas the AREDS Study showed no benefit of antioxidant supplements for early ARM. Similarly, results from the Rotterdam Study showed a decreased risk for early ARM in subjects with high dietary intake of vitamin E or zinc, by comparison with those with low intake(29). A European supplementation study would be needed to better assess the benefit of antioxidant supplementation in European populations.
A more recent research domain evaluated the role of two carotenoids, lutein and zeaxanthin, in the protection of the retina and the lens. These carotenoids accumulate in the macula, where they are known as the macular pigment(30). Besides their antioxidant properties, they probably act as a filter against the phototoxic effects of blue light(30). To date, five epidemiological studies have assessed the associations of the risk of AMD with plasma concentration of lutein and zeaxanthin(31-35). As shown in Fig. 1, all five studies showed a decreased risk for AMD in subjects with high plasma concentrations of lutein and zeaxanthin, although the association was statistically significant only in 2 studies(32-35). With regard to dietary intake, four prospective population-based studies were published(29,36-38).
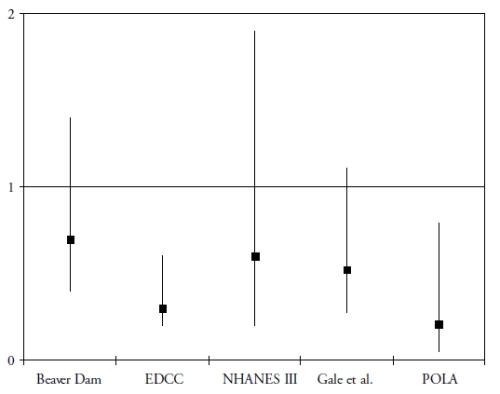
Figure 1. Association of the risk of AMD with plasma levels of lutein and zeaxanthin in cross-sectional and case-control studies (odds-ratios with 95% confidence interval)
OR below 1 suggest a protective role and OR greater than 1 suggest a deleterious role. References of the cited studies: Beaver Dam(31); EDCC(32); NHANES III(33); Gale et al(34); POLA(35)
These studies assessed the risk for developing AMD (in subjects initially free of AMD), according to their dietary intake of lutein and zeaxanthin. As shown in Fig. 2, the results for these dietary studies are less clear than for those on plasma measurements.
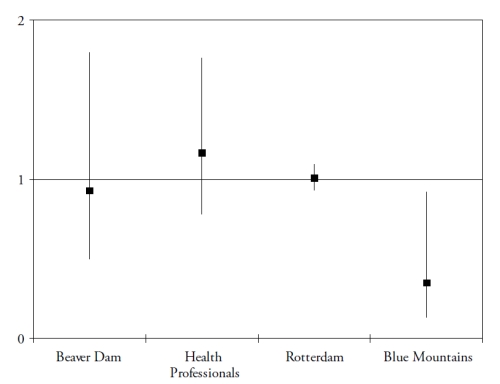
Figure 2. Associations of the risk for AMD with dietary lutein and zeaxanthin, in published epidemiological prospective studies. References of the cited studies: Beaver Dam(36); Health Professionals(37); Rotterdam(29); Blue Mountains(38).
Only one study found a significantly reduced risk for AMD in subjects with high dietary intake of lutein and zeaxanthin(38). However, dietary assessment methods rely on the subjects’ memory and perceptions and face the difficulties of the extreme day-to-day variability of human diet, the bias in reporting due to social standards and nutritional recommendations and the estimations of nutritional contents of food items. Biomarkers have the advantages of being objective, and of taking into account individual variations in bioavailability and metabolism. For instance, smoking and obesity are known to decrease the bioavailability of carotenoids(39-40). Despite normal dietary intake in lutein and zeaxanthin, subjects may be at higher risk for AMD because of decreased bioavailability, associated with lower plasma concentrations of these components. However, currently available studies including plasma measurements are cross-sectional or case-control studies, where the plasma measurements were performed in subjects already affected by the disease. The stronger associations found in these studies may therefore be explained by reverse causality (i.e. plasma carotenoids were lower because of change of dietary habits in subjects with AMD, for instance).
Globally, the few available epidemiological studies suggest a protective role of lutein and zeaxanthin in AMD, but other studies are needed in this field, in particular larger, prospective studies including dietary and plasma measurements.
Finally, omega 3 PUFA include a precursor (alpha-linolenic acid (ALA)), and three long-chain derivatives (eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA)). ALA is an essential nutrient, since humans cannot synthesize it de novo and therefore rely on diet as its sole source. Synthesis of the long-chain derivatives is very limited in humans(41), who must therefore rely on their dietary supply, mainly by fish and seafood. Long-chain omega-3 PUFA have important structural and protective functions in the retina. DHA is a major component of the photoreceptors(42). They also have protective functions, including the systemic anti-inflammatory, anti-angiogenic and anti-apoptotic functions and specific actions in the retina such as increase in lysosomial acid lipase, leading to increased lipid degradation in the retinal pigment epithelium(42). As shown in Fig. 3 and Fig. 4, seven case-control(43-44) or cross-sectional studies(45-49) and five prospective studies(50-54) assessed the associations of dietary intake of long-chain omega 3 PUFA or fish with AMD. In spite of differences in populations, methods and types of studies, all studies showed a reduced risk for AMD in subjects with high dietary intake of long chain omega 3 PUFA or fish, although some of these associations did not reach statistical significance.
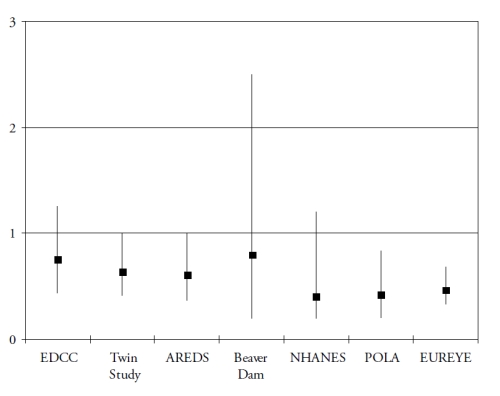
Figure 3. Association of the risk for AMD with dietary long chain omega 3 or fish (cross-sectional and case-control studies). References of the cited studies: EDCC(43); Twin Study(45); AREDS(44); Beaver Dam(46); NHANES(47); POLA(48); EUREYE(49)
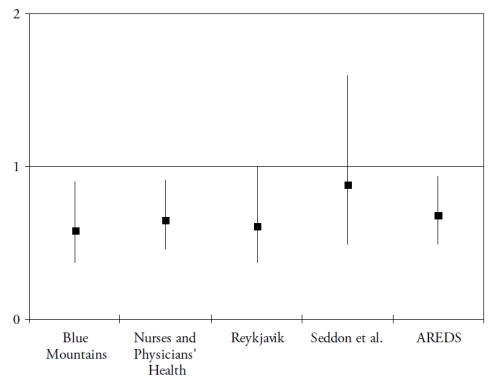
Figure 4. Association of the risk for AMD with dietary long chain omega 3 or fish (prospective studies). References of the cited studies: Blue Mountains(51); Nurses and Physicians’Health Study(50); Reykjavik Study(52); Seddon et al.(53); AREDS(54)
Most of these studies were grouped in a meta-analysis performed in 2008, which concluded at a 38% reduction in risk for AMD in subjects with high dietary long-chain omega 3 PUFA(55). By contrast, two recent studies found an increased risk for ARM in subjects with high omega 3 PUFA(56-57). However, in these studies, only total omega 3 PUFA intake was studied, including ALA and long-chain omega 3 PUFA, while most studies found a reduced risk for ARM only with high long chain omega 3 PUFA, in accordance with the scientific rationale. As stated in one of these studies(57), the main source for ALA is vegetable oil, which is also the main source of omega 6 PUFA, and was found to increase the risk for ARM in some studies(43,53). Future studies need to separate the precursor from the long chain derivatives.
Globally, available epidemiological studies strongly suggest a reduced risk for AMD in subjects with high consumption of long chain omega 3 fatty acids and fish.
In addition, some studies have suggested that the risk for AMD may be decreased in subjects with high intake of vitamins B(56-63). Consistently, supplementation with vitamins B reduced the incidence of AMD in a randomized interventional study(64). A role for vitamin D in the aetiology of AMD has also been suggested(65).
4. Light exposure and cataract extraction
Sunlight exposure has been investigated as a potential risk factor for AMD. Light exposure may have deleterious effect on the eye, in particular through the production of reactive oxygen species(66). Only blue light reaches the retina, since ultraviolet radiations are absorbed by the cornea and the lens. Intense blue light exposure has been shown to induce retinal damage(66), and the macular pigment, which absorbs blue light, is thought to protect the macula against photo-toxic damage(30). However, results have been inconsistent in the few studies that have investigated the associations of the light exposure with AMD in humans(27,67-74). Globally, the available studies suggest that the effect of sunlight exposure in the aetiology of AMD is at most modest. Interestingly, a recent study evidenced an association of the risk for AMD with blue light exposure, only in those subjects with low plasma antioxidants and zeaxanthin(27). This suggests that light exposure may increase the risk for AMD only when defences against the produced reactive oxygen species are not appropriate. Sunlight exposure therefore does not appear to be a major determinant of AMD, but may be a risk factor in susceptible individuals. Appropriate nutritional intake in antioxidants and macular pigment may be particularly important in subjects highly exposed to light. These data will need to be confirmed in future studies.
Besides, cataract surgery was associated with a major increase in AMD incidence in a few studies(7,75-81), although not all(82-83). For instance, in a pooled analysis of two major population-based studies (Beaver Dam and Blue Mountains), eyes which had undergone lens extraction had a 5.7-fold increased risk of developing late AMD(79). The reasons for increased risk of AMD in aphakic and pseudophakic eyes are unknown, but may include increased light exposure. Indeed, the lens naturally absorbs ultraviolet light, and, with the lens yellowing observed with ageing and cataract, also part of blue light. In this context, use of blue light filters in the implanted artificial lenses have been proposed(84), and are currently widely used, although their potential effect on the reduction of incidence of AMD has not been evaluated.
Because cataract surgery is the most frequent surgical procedure in most industrialized countries, the potential increased risk of AMD in operated eye needs further study, in order to better characterize it, to determine its causes and to identify strategies to limit this potentially deleterious effect.
In conclusion, AMD is emerging as a disease resulting from major genetic susceptibility, the effect of which is modulated by lifestyle. Among lifestyle factors, smoking is the best characterized risk factor, now considered as causal, while the role of nutrition is increasingly identified. The respective roles of antioxidants, macular pigment and omega 3 fatty acids, together with other potential nutritional factors such as vitamins B and D, will be better understood in the future. In this field, several new epidemiological studies are being conducted, among which, in France, the Alienor Study(85). This population-based cohort study aims at assessing the association of AMD with nutritional, vascular and genetic risk factors. It bears on almost 1000 subjects, recruited from an existing cohort study on brain ageing (the 3C Study). The main nutritional factors studied are lutein and zeaxanthin, antioxidants (vitamins C, E, zinc) and omega 3 fatty acids, and are measured both in the diet, in plasma and, for lutein and zeaxanthin, on the retina. This study will add to the existing literature in this field, which is still relatively scarce and partially inconsistent. Moreover, several large controlled interventional trials, including the AREDS2 Study, will help demonstrating their causal role in the aetiology of AMD. Finally, the role of light exposure (in particular blue light) does not seem to be major determinant in this disease, but may be important in subgroups of the population (subjects with low antioxidant and macular pigment intake, subjects undergoing cataract surgery).