Updated/reviewed by the authors, July 2017.
Development and Progression of AMD
Author:
Maria Luz Cachulo, MD PhD; José Costa, MD Msc
Coimbra University Hospital - Coimbra, Portugal
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in older population in industrialized nations(1,2). It is characterized by ageing changes at the photoreceptors, retinal pigment epithelium (RPE), Bruch’s membrane and choroid(3,4).
The clinical hallmark of the early stage of AMD is drusen, which are yellowish deposits at the level of the RPE. Focal RPE hyperpigmentation and atrophy can also be seen. The advanced stage of non-neovascular AMD is Geographic Atrophy (GA) where areas of atrophy become confluent and cause visual loss. Neovascular AMD (NV-AMD) is also an advanced manifestation of AMD and is characterized by choroidal neovascularization (CNV).
This review summarizes the various biomarkers of AMD and analyses whether or not they may, one day, be exploited to determine risks of disease onset, measure progression of disease or even assess the effects of treatment of AMD.
Environmental Risk Factors
See chapter “Modifiable Risk Factors for AMD”.
Genetic biomarkers
Genetic predisposition for AMD has been demonstrated by familial aggregation studies and twin studies(5,6). Using genome linkage scan and association studies, multiple potentially causative genes and/or single nucleotide polymorphisms (SNPs) have been identified(7-9).
The most commonly implicated genetic risk loci are located in chromosome 1q25-31, which are related to several complement system regulators, such as complement factor H (CFH) and CFH-related proteins (CFHR1-5)(10). CFH, in particular, is a key regulator of the complement system of innate immunity: it maintains the homeostasis of the complement system and protects bystander host cells and tissues from damage by complement activation(11). At least 6 genetic variants related to AMD development in Caucasians have been identified(7,12-14), while only three of these have been described in Chinese and Japanese populations(15,16).
The most documented risk-conferring single-nucleotide polymorphism results is a tyrosine-to-histidine substitution in 1q26 at position 402 of the CFH protein. This CFHY402H polymorphism increases AMD risk 2 to 4-fold in heterozygotes and 3 to 7-fold in homozygotes(7,17). It causes a significant loss of binding of factor H to heparin, C-reactive protein and to peroxidised lipids on dead cells combined with reduced anti-inflammatory functions by factor H, leading to increased local complement activation(18).
The CFHR genes comprise five plasma proteins (CFHR1-5) that bind to the central complement component C3b. These are located at 1q32 within the RCA (Regulation of Complement Activation) gene cluster(10). A homozygous deletion of CFHR1 and CFHR3 is a relatively common finding in several populations and appears to confer a lower risk of AMD(19). A possible explanation for the protective effect of these deletions might be that CFHR1 and CFHR3 compete with CFH for binding to C3b, thus a deficiency of these proteins increases the efficacy of factor H and its regulatory effect on complement activation(10). The prevalence of this deletion differs between ethnic groups, occurring in 17.3% of Africans, 15.9% of Afro-Americans, 6.8% of Hispanics, 4.7% of Caucasians and 2.2% of Chinese. This finding is consistent with the AMD prevalence rates, which are lower in African-Americans than in Caucasians and Chinese(12).
For an in-depth review of genetic biomarkers of AMD, please refer to the chapter “Genetics of AMD”.
Inflammatory biomarkers
Besides components of the complement system(20), several immunological molecules and inflammatory mediators have been identified at the site of AMD lesions(21). In fact, chronic inflammation appears to be a causative factor for the development of AMD by causing endothelial dysfunction and facilitating interactions between modified lipoproteins, monocyte-derived macrophages, T-cells and normal cellular elements of the retinal vasculature(22). Activated macrophages and microglia may cause cellular damage, Bruch’s membrane degradation and angiogenesis by secreting chemokines and cytokines(22). In fact, using electron microscopy or immunohistochemistry methods, macrophages can be found in the area of geographic atrophy phagocytising pigment debris(23) and around CNV in wet AMD(24).
In order to grasp the role of inflammation on the development and progression of AMD, several markers of systemic inflammation have been extensively studied. C-reactive protein (CRP), an acute phase serum protein, is a surrogate for interleukin-6 (IL-6)(25). Classically seen as an inflammatory marker, CRP is now regarded as an independent risk for both cardiovascular and peripheral arterial disease(26). It directly upregulates endothelial cell adhesion molecules and promotes the release of chemoatractant chemokines, which have a negative effect on the retinal microvasculature(22,27).
A meta-analysis of data from more than 41.000 patients found a two-fold increase of late AMD risk in patients with CRP higher than 3 mg/L when compared to those with serum levels <1mg/L(28). The effect of CRP levels on AMD progression might be due to chronic inflammation leading to oxidative damage, endothelial dysfunction, drusen development and the degeneration of Bruch’s membrane(29). Additionally, CRP may also have a direct role in AMD development through its ability to induce complement activation(30).
IL-6 is a marker for systemic inflammation, such as acute pancreatitis and chronic arthritis. It has been implicated in angiogenesis, along with VEGF, in several in vitro and cancer studies(31). Seddon and colleagues found a correlation between the level of IL-6 and chances of AMD progression(32). Animal studies have shown that CNV induction by laser stimulated IL-6 expression in the RPE–choroid complex, and that blockade of IL-6 led to a significant suppression of CNV(33). Interestingly, high aqueous IL-6 levels might to predict resistance to intravitreal bevacizumab in patients with wet AMD(34).
Fibrinogen is also an established biomarker of acute and chronic inflammation(35). Lip and colleagues found elevated levels of plasma fibrinogen in AMD cases compared with controls(36), and a case–control analysis from the large Blue Mountains Eye Study in Australia detected significantly elevated plasma fibrinogen levels in AMD patients compared with controls (p < 0.05)(37). In another study using patients recruited from the Muenster Aging and Retina Study population in Münster (Germany), plasma fibrinogen levels were found to be elevated as the degree of AMD severity increased(38). In fact, fibrinogen plasma levels appear to be particularly increased in patients with exudative AMD(39).
Multimodal imaging evaluation of AMD progression
1) Fundoscopy and Colour Fundus Photographs
Despite the increasingly sophisticated imaging techniques available, fundoscopy and colour fundus photographs (CFP) remain invaluable tools for the assessment of the severity and risk of progression of patients with AMD.
Drusen size, area and location are important predictors of AMD progression(40,41): large soft drusens with ill-defined borders and extensive drusen area are the most important risk factors for the progression to advanced AMD identifiable on CFP(42,43). Yet, earlier macular changes can provide valuable information about the risk of AMD progression. This has been extensively demonstrated in population-based observational studies and provides the basis for several AMD severity classifications.
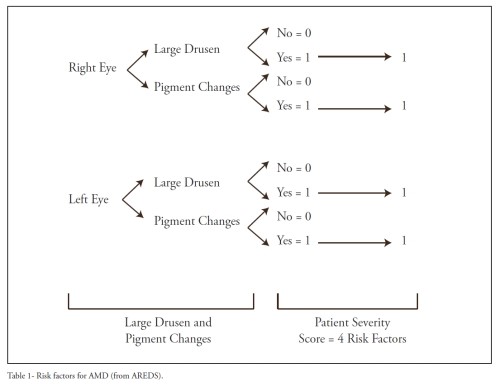
The Age-Related Eye Disease Study (AREDS) collected stereoscopic CFP of more than 3000 AREDS over 5 years of follow-up and identified large drusen and macular pigmentary abnormalities as features that, cumulatively, increase the risk of progression to advanced AMD(43). A simplified risk scale was developed based on these findings (Table 1). It considers the presence of at least 1 large drusen (diameter greater than or equal to that of a large vein at the disc margin) and the presence of any pigment abnormality as 1 point each, and sums their presence across both eyes when both are free of advanced AMD. One point is assigned to patients who have no large drusen in either eye but intermediate-sized drusen (diameter ≥ one half that of a large drusen) in both eyes. The 5-year risk of advanced AMD using this scale increases as more risk factors are present: 0 factors, 0.5%; 1 factor, 3%; 2 factors, 12%; 3 factors, 25%; and 4 factors, 50%. This simplified scale is particularly useful in clinical practice because it allows fast risk stratification and is useful when discussing with patients their risk of progression to vision-threatening AMD.
In recent years, several studies have studied the significance of reticular pseudodrusen (RPD). They were first described in 1990 as a yellowish interlacing network most commonly located on the superotemporal macula(44). RPD are difficult to identify in clinical fundoscopic examination and are poorly visualized with regular CFP, so they are best seen with multimodal imaging(45). In two population-based studies, the overall prevalence of RPD was 0.7%-1.95% for the general population(45,46). In AMD patients, the prevalence of RPD is significantly higher: they are present in up to 58% of patients(47,48). This finding is particularly relevant due to the fact that RPD are associated with greater risk of progression to advanced AMD: the presence of RPD at baseline in fellow eyes of patients with unilateral neovascularization is a significant predictor of progression to advanced AMD (OR =2.5) over a 5-year period, particularly the wet form(48). Similarly, depending on the follow-up, up to 14% of patients with AMD ultimately develop GA(47-49). Unfortunately, RPD presence remains an underreported and underresearched retinal phenotype, but the advent of new imaging modalities will certainly improve our knowledge about this phenotype(48).
2) Fundus Autofluorescence
Fundus autofluorescence (FAF) is a non-invasive method that supplies additional information to that obtained using CFP and fluorescence angiography(50). It provides an indirect evaluation of RPE function, an important component of the physiopathology of AMD(51). In GA, the atrophic areas appear as hypoautofluorescent patches surrounded by non-atrophic retina of variable autofluorescence(52). The FAF features of these borders of GA lesions appear to be particularly important to predict the progression of pre-existing GA, as variation in GA growth rates are dependent on the specific phenotype of FAF at baseline(53-55).
Additionally, FAF has a great sensitivity for the detection of RPD: areas covered with RPD usually show a reticular FAF pattern with small areas of decreased FAF surrounded by normal FAF(55).
For further data on FAF, please refer to the chapter “Fundus autofluorescence in age-related macular degeneration”.
3) Optical Coherence Tomography
Due to the quantitative and highly reproducible data that it provides, optical coherence tomography (OCT) has quickly become an invaluable tool to evaluate AMD progression and treatment response.
Spectral domain OCT (SD-OCT), in particular, enables automated measurement of drusen area and volume(41), important predictors of AMD progression(56), and has been validated as a method of following GA development and progression(57). In addition, RPD can be easily seen on SD-OCT B-scans as triangular shaped structures located above the RPE line(58).
Detailed information about the role of OCT in the management of AMD can be found on the chapter “Optical Coherence Tomography in Age-related Macular Degeneration”.
Conclusion
AMD is a complex disease caused by a combination of genetic predisposition and environmental factors and the interplay between these remains a mystery. A better understanding of the involved pathophysiologic process or the identification of biomarkers for the conversion would enhance our ability to diagnose and treat the wet AMD, to develop better therapies and, eventually, to prevent vision loss associated with the disease.