Epidemiology of AMD
Epidemiology of AMD
Author:
Cécile Delcourt, MD, PhD
Inserm, U897, Bordeaux, France Université Bordeaux 2, Bordeaux, France
1. Introduction
Although age-related macular degeneration (AMD) is the third cause of blindness worldwide, and the first in industrialized countries(1), epidemiological data on this disease remain scarce and partial. The very first studies were published in the 1980’s. Since then, a number of population-based studies have been conducted, first in Caucasian populations of the United States and other industrialized countries (Australia, Europe). Studies have more recently been extended to other ethnical subgroups of industrialized countries (African Americans, Latinos) and to other parts of the world (India, China, and Japan).
2. Prevalence of late AMD in Caucasians from industrialized countries
The prevalence of late AMD in the main epidemiological studies performed in Caucasians of industrialized countries are presented in Table 1.
|
Author |
Study Years study conducted |
Country Number of subjects, age |
Prevalence of late AMD(%) |
|
United States |
|
|
|
|
Friedman, Baltimore Eye Study(28) |
1985-1988, USA |
N=2518, age >= 40 years |
1.23 |
|
Klein, Beaver Dam Eye Study(29) |
1988-1990, USA |
N=4752, age >= 40 years |
1.64 |
|
Bressler, Salisbury Eye Evaluation Project(30) |
1993-1995, USA |
N=1773, age >= 65 years |
Exudative AMD : 1.7 Geographic atrophy: 1 |
|
Klein, Atherosclerosis Risk in Communities Study(31) |
1993-1995, USA |
N=8984 , age 48-72 years (one eye only) |
0. |
|
Klein, Cardiovascular Health Study(32) |
1997-1998, USA |
N=1998, age 69-97 years (one eye only) |
1.3 |
|
Klein, Multi-Ethnic Study of Atherosclerosis(10) |
2000-2002, US |
N=2315, age 45-84 years |
0.6 |
|
Australia |
|
|
|
|
Mitchell, Blue Mountains Eye Study(33) |
1992-1994, Australia |
N=3632, age >= 50 |
2.06 |
|
Van Newkirk, Melbourne VIP(34) |
1992-1996, Australia |
N=4345, age >= 40 years |
0.68 |
|
Europe |
|
|
|
|
Vingerling, Rotterdam Study(35) |
1990-1993, Netherlands |
N=6774, age >= 55 years |
1.65 |
|
Vingerling, Rotterdam Study(35) |
1995-1997, France |
N=2196, age >= 60 years |
1.9 |
|
Jonasson, Reykjavik Eye Study(5) |
1996, Iceland |
N=1022, age >= 50 years |
3.5 |
|
Topouzis, Thessaloniki Eye Study(37) |
2000, Greece |
N=1022, age >= 50 years |
2.5 |
|
Bjornsson, Oslo Macular Study(6) |
2002, Norway |
N=459, age > 50 years |
2.8 |
|
Augood, Eureye(4) |
2001-2002, |
N=4753, age >= 65 years |
3.3 |
|
|
7 european countries |
|
|
Table 1. Prevalence of late AMD in Caucasians from industrialized countries
The included studies were restricted to those having classified AMD from retinal photographs and used the international classification(2), in order to make comparisons between studies easier. In these studies, late AMD is defined by the presence of neovascular AMD and/or geographic atrophy. In the United States, the prevalence of late AMD ranged from 0.2% to 1.6% according to studies. Since the prevalence of late AMD increases sharply with age, most of the differences between studies were due to differences in the age distribution. For instance, the lowest prevalence rate (0.2%) was observed for the Atherosclerosis Risk in Communities (ARIC) Study. In this study, the age range of participants was 48-72 years, thus excluding the oldest subjects, in which the prevalence of late AMD is the highest. This study also performed photographs in only one eye of each participant, which may have led to undetected unilateral cases and thus underestimation of the prevalence of late AMD. This is also the case for the Cardiovascular Health Study, which also found relatively low prevalence (1.3% in subjects aged 69 to 97 years). Prevalence rates observed in Caucasians from Australia and Europe appear relatively similar to those observed in the United States (Table 1), when taking into account differing age distributions in the different studies. In European studies, the prevalence of late AMD ranged from 1.65% to 3.5%.
In order to better compare the prevalence rates among studies, age-specific prevalence rates need to be estimated. This has been done in a meta-analysis performed in 2004 by Friedman et al(3). The authors concluded that prevalence rates were not different among Caucasian populations of industrialized countries, including the United States, Australia and the Netherlands. Similarly, in the EUREYE Study, which included 7 European countries (Norway, Estonia, Ireland, France, Italy, Greece, Spain), no significant differences were found among the participating countries(4). Therefore, the prevalence of late AMD appears to be similar in the Caucasian populations from the United States, Australia and European countries, despite major geographical and lifestyle differences. Thus, the meta-analysis from Friedman et al. probably constitutes the most reliable available estimate of prevalence rates for these countries, since it bears on studies gathering altogether more than 25000 subjects(3). In this meta-analysis, the prevalence rates increase sharply with age (Figure 1), from less than 0.5% in subjects 50 to 60 years, to 12% and 16% in men and women aged 80 years or more, respectively. While men tend to have higher prevalence rates than women in the younger age groups (less than 80 years), late AMD is more frequent in women than in men in the oldest age group (80 years or more). This may be due to the higher mortality in ageing men, which leads to a higher selection of the oldest subjects. Finally, in the late AMD groups, two clinical entities exist: neovascular AMD and geographic atrophy. In most studies, neovascular cases represent about 55% of all late AMD cases(3). Nordic European countries (in particular Iceland and Norway) may be an exception, since the few studies performed in these countries suggest a higher rate of geographic atrophy(5,6).
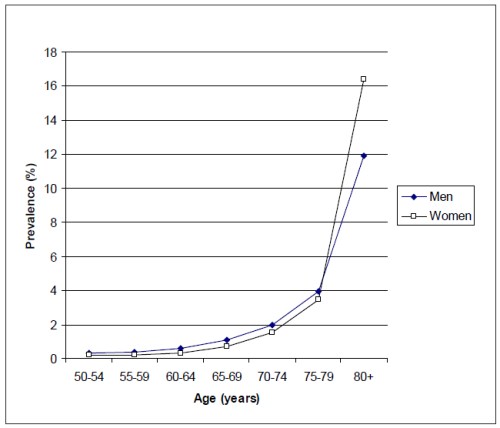
Figure 1. Prevalence of late AMD according to age and gender in Caucasians from industrialized countries (adapted from the meta-analysis by Friedman et al.(3))
3. Prevalence of late AMD in other ethnic groups
As shown in Table 2, the prevalence of late AMD is much lower in African-Americans than in Caucasians.
|
Author |
Study Years study conducted |
Country Number of subjects, age |
Prevalence of late AMD(%) |
|
African-Americans |
|
|
|
|
Friedman, Baltimore Eye Study(28) |
1985-1988, USA |
N=1843, age >= 40 years |
0.22 |
|
Bressler, Salisbury Eye Evaluation Project(30) |
1993-1995, USA |
N=666, age >= 65 years |
Exudative AMD : 1.1 Geographic atrophy : 0.3 |
|
Klein, Atherosclerosis Risk in Communities |
1993-1995, USA |
N= 2548 , age 48-72 years (one eye only) |
0.04 |
|
Study(31) |
|
|
|
|
Klein, Cardiovascular Health Study(32) |
1997-1998, USA |
N=363, age 69-97 years (one eye only) |
0.3 |
|
Hispanics |
|
|
|
|
San Luis Vally(17) |
1983, USA |
N=3995, age 43-74 years |
0.09 |
|
Proyecto VER(15) |
1997-1999, USA |
N=2776, age >= 50 years |
0.5 |
|
Los Angeles Latino Eye Study(16) |
2000-2003, USA |
N=5875, age >= 40 years |
0.43 |
|
Klein,Multi-Ethnic Study of Atherosclerosis(10) |
2000-2002, USA |
N=1280, age 45-84 years |
0.2 |
|
Chinese |
|
|
|
|
Klein, Multi-Ethnic Study of Atherosclerosis(10) |
Klein, Multi-Ethnic Study of Atherosclerosis(10) |
N=699, age 45-84 years |
1.0 |
Table 2. Prevalence of late AMD in other ethnic groups from the United States
Most of the cited studies were multi-ethnic and performed direct comparisons between African-Americans and Caucasians. Statistical power was generally low for these comparisons, but these studies globally suggest that the prevalence of late AMD is at least 2-fold lower in African-Americans. In the Barbados Eye Study, bearing on subjects of African origin from the Barbados Island, the prevalence rate of late AMD (0.57%) was similar to those observed in African-Americans (Table 3)(7).
|
Author |
Study Years study conducted |
Country Number of subjects, age |
Prevalence of late AMD(%) |
|
Barbados |
|
|
|
|
Schachat, Barbados Eye Study(7) |
11987-1992, Barbados |
N=3444, age 40-84 years |
0.57 |
|
Japan |
|
|
|
|
Oshima, Hisayama Study(8) |
1998, Japan |
N=889, age >= 50 years |
0.89 |
|
Kawasaki, Funagata Study(9) |
200-2002, Japan |
N=1625, age >= 35 years |
0.5 |
|
|
|
N=1037, age >= 55 years |
0.8 |
|
India |
|
|
|
|
Krishnaiah, Andrah Pradesh Study(38) |
1996-2000, India |
N=3723, age >= 40 years |
1.9 |
|
Nirmalan, Aravind Eye Study(18) |
1995-1997, India |
N=4197, age >= 40 years |
0.6 |
|
Krishnan, INDEYE(19) |
2005-2007, India |
N=4266, age >= 60 years |
1.2 |
|
China |
|
|
|
|
Li, Beijing Eye Study(11) |
2001, China |
N=4376, age >= 40 years |
0.2 |
|
Taïwan |
|
|
|
|
Chen, Shihpay Study(12) |
1999-2000, Taïwan |
N=1105, age >= 65 years |
1.9 |
|
Singapore |
|
|
|
|
Kawasaki, Singapore Malay Eye Study(13) |
2004-2006, Singapore |
N=3265, age 40-80 years |
0.7 |
|
Greenland |
|
|
|
|
Andersen, Greenland Inuit Eye Study(14) |
2002-2003, Greenland |
N=642, age >= 60 years |
9.1 |
Table 3. Prevalence of late AMD in other countries
Therefore, people of African origin appear to be at much lower risk of late AMD than Caucasians, although no data are available from African countries. American Hispanics also appear to be at lower risk for late AMD than Caucasian, with prevalence rates ranging from 0.09% to 0.5% among studies (Table 2). The reasons for the lower prevalence rates in African-American and Hispanics are unclear. No data are available on minorities of other industrialized countries, in particular in Europe. This constitutes a limitation to the estimation of the prevalence of AMD in European countries, which have different types of ethnic groups than in the United States (populations originating from North Africa, Middle-east countries, India…). Given the major differences observed in ethnic groups in the United States, such epidemiological data in European minorities are warranted.
Other epidemiological studies have mainly been conducted in Asia (Japan, India and China). In contrast to what was originally thought, late AMD is not rare in these Asian populations, as shown in Table 3. In two Japanese studies(8,9), the prevalence rates in Japanese men were similar to those observed in Caucasian men from industrialized countries, while late AMD appeared rare in Japanese women. This gender-effect, which is not observed in industrialized countries, may be due to gender-related smoking habits in Japanese. Indeed, smoking is a major risk factor for AMD, in all studied populations. In these two studies, smoking was very frequent in Japanese men, while rare in Japanese women.
In three epidemiological studies performed in India, the prevalence rates of late AMD ranged from 0.6% to 1.9% (Table 3). Below the age of 80 years, the prevalence rates were rather similar to those observed in industrialized countries. Above 80 years, the figures are mostly unreliable because of the very high prevalence of unoperated cataracts in this population, leading to a very high percentage of ungradable retinal photographs. The global prevalence rates were therefore probably underestimated.
Three studies have been performed in subjects of Chinese origin: one in the United States (Table 2)(10), one in mainland Beijing(11) and one in Taïwan(12). While prevalence rates in Chinese from the United States and Taïwan seemed similar to those in Caucasians, the prevalence rates in Chinese from Mainland China were much lower.
The reasons for these differences are unclear, and may be related to gene-environment interactions, since Chinese from the United States and Taïwan tend to live a more industrialized lifestyle. More epidemiological data are needed to confirm and understand the reasons for these differences.
Finally, in a study from Singapore, the prevalence of late AMD was similar to that in Caucasians(13). Globally, the prevalence of late AMD appears high in Asians, with the only exception of Chinese from Mainland China, which remains to be confirmed.
A study in Inuits from Greenland shows strikingly high rates of late AMD, with particularly high rates of neovascular AMD(14). This is in contrast from the observations of European Nordic countries, which found higher rates of geographic atrophy(5,6) and also in contrast with original observations in this population, which also found higher rates of geographic atrophy in Inuits. The reasons for these differences are unclear.
4. Prevalence of early AMD
Late AMD is preceded by early, usually asymptomatic, retinal abnormalities. The long-term cohort studies have helped to characterize the lesions that are the most predictive of incident late AMD. While large soft drusen (>125 microns) and pigmentary abnormalities clearly are the hallmarks of early AMD, there is no consensus on the precise definition of early AMD, and several classification systems coexist. This makes it difficult to assess and compare the prevalence of early AMD among geographical areas and ethnic groups.
Despite these difficulties, it appears that early AMD is frequent in the elderly. For instance, in the meta-analysis by Friedman et al, the prevalence of large drusen increased from about 1.5% in Caucasians aged 40-49 years, to more than 25% in those aged 80 years or more(3). Similar rates were observed in the EUREYE Study, performed in Europe(4).
Consistently with what was observed for late AMD, early AMD appears less frequent in African-Americans than in Caucasian(3,10). By contrast, while late AMD is also less frequent in Hispanic-Americans, the prevalence of early abnormalities appears similar, or even higher than in Caucasians(10,15-17). The reasons for these differences are unclear, and suggest that different factors may be implicated in the etiology of early and late AMD. In most studies performed in Asians, the prevalence of early AMD appears similar to that observed in Caucasians, consistently to what was found for late AMD(8-10,12,13,18,19).
5. Prevalence of late AMD in Portugal
To our knowledge, there are no published data on the prevalence of AMD in Portugal. What can therefore be extrapolated from available data in other countries? As explained above, the prevalence of late AMD appears to be similar in Caucasian populations from industrialized countries. Its best estimate therefore derives from the meta-analysis by Friedman et al(3). We, thus, applied the age and gender-specific prevalence rates of the meta-analysis to the demographic data from Portugal, leading to an estimation of about 84 000 cases of late AMD in Portugal (Table 4).
|
Population of Portugal |
AMD prevalence (%) * |
Number with AMD |
|
|
Men |
|
|
|
|
50-54 |
309 484 |
0,34 |
1 052 |
|
55-59 |
268 899 |
0,41 |
1 102 |
|
60-64 |
256 179 |
0,63 |
1 614 |
|
65-69 |
244 230 |
1,08 |
2 638 |
|
70-74 |
196 615 |
1,98 |
3 893 |
|
75-79 |
143 439 |
3,97 |
5 695 |
|
80+ |
123 934 |
11,90 |
14 748 |
|
Total men |
1 542 780 |
1,99 |
30 742 |
|
|
|
|
|
|
Women |
|
|
|
|
50-54 |
333 032 |
0,20 |
666 |
|
55-59 |
302 553 |
0,22 |
666 |
|
60-64 |
294 737 |
0,35 |
1 032 |
|
65-69 |
293 935 |
0,70 |
2 058 |
|
70-74 |
257 347 |
1,52 |
3 912 |
|
75-79 |
204 627 |
3,44 |
7 039 |
|
80+ |
229 366 |
16,39 |
37 593 |
|
Total women |
1 915 597 |
2,76 |
52 965 |
|
Total |
3 458 377 |
2,42 |
83 707 |
Table 4. Prevalence of late AMD in Portugal according to age and gender
A major limitation to this approach is that it does not take into account the prevalence rates of AMD in non Caucasians living in Portugal. As shown above, the prevalence of late AMD appear lower in subjects of African origin. However, no data are available on the prevalence of AMD in subjects of African, or other ethnic origins (for instance North Africa and Asia) in European populations. It appears difficult to assess the prevalence of AMD while taking into account the multi-ethnic structure of the Portuguese population. However, these ethnic subgroups represent small minorities in Portugal, so that this may only marginally affect the global prevalence rates.
According to these estimations, about two thirds of cases are women, and two thirds of cases are aged 80 years or more. Since it represents about 55% of late cases, neovascular AMD probably affects about 46 000 subjects.
References - Epidemiology of AMD
References - Epidemiology of AMD
1. Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82 (11): 844-51.
2. Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, Klein R, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995; 39 (5): 367-74.
3. Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 122 (4): 564-72.
4. Augood CA, Vingerling JR, de Jong PT, Chakravarthy U, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Bentham G, Rahu M, Vioque J, Young IS, Fletcher AE. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol 2006; 124 (4): 529-35.
5. Jonasson F, Arnarsson A, Sasaki H, Peto T, Sasaki K, Bird AC. The prevalence of age-related maculopathy in iceland: Reykjavik eye study. Arch Ophthalmol 2003; 121 (3):379-85.
6. Björnsson OM, Syrdalen P, Bird AC, Peto T, Kinge B. The prevalence of age-related maculopathy (ARM) in an urban Norwegian population: the Oslo Macular study. Acta Ophthalmol Scand 2006; 84 (5): 636-41.
7. Schachat AP, Hyman L, Leske MC, Connell AM, Wu SY. Features of age-related macular degeneration in a black population. The Barbados Eye Study Group. Arch Ophthalmol 1995; 113 (6): 728-35.
8. Oshima Y, Ishibashi T, Murata T, Tahara Y, Kiyohara Y, Kubota T. Prevalence of age related maculopathy in a representative Japanese population: the Hisayama study. Br J Ophthalmol 2001; 85 (10): 1153-7
9. Kawasaki R, Wang JJ, Ji GJ, Taylor B, Oizumi T, Daimon M, Kato T, Kawata S, Kayama T, Tano Y, Mitchell P, Yamashita H, Wong TY. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: the Funagata study. Ophthalmology 2008; 115 (8): 1376-81, 1381.e1-2.
10. Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, Burke G, Saad MF, Jacobs DR Jr. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006; 113 (3): 373-80.
11. Li Y, Xu L, Wang YX, You QS, Yang H, Jonas JB. Prevalence of age-related maculopathy in the adult population in China: the Beijing eye study. Am J Ophthalmol 2008; 146 (2): 329.
12. Chen SJ, Cheng CY, Peng KL, Li AF, Hsu WM, Liu JH, Chou P. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci 2008; 49 (7): 3126-33.
13. Kawasaki R, Wang JJ, Aung T, Tan DT, Mitchell P, Sandar M, Saw SM, Wong TY; Singapore Malay Eye Study Group. Prevalence of age-related macular degeneration in a Malay population: the Singapore Malay Eye Study. Ophthalmology 2008; 115 (10): 1735-41. 14. Andersen MV, Rosenberg T, la Cour M, Kiilgaard JF, Prause JU, Alsbirk PH, Borch-Johnsen K, Peto T, Carstensen B, Bird AC. Prevalence of age-related maculopathy and age-related macular degeneration among the inuit in Greenland. The Greenland Inuit Eye Study. Ophthalmology 2008; 115 (4): 700-707.e1. 15. Muñoz B, Klein R, Rodriguez J, Snyder R, West SK. Prevalence of age-related macular degeneration in a population-based sample of Hispanic people in Arizona: Proyecto VER. Arch Ophthalmol 2005; 123 (11): 1575-80.
16. Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP; Los Angeles Latino Eye Study Group. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology 2004; 111 (7): 1288-97.
17. Cruickshanks KJ, Hamman RF, Klein R, Nondahl DM, Shetterly SM. The prevalence of age-related maculopathy by geographic region and ethnicity. The Colorado-Wisconsin Study of Age-Related Maculopathy. Arch Ophthalmol 1997; 115 (2): 242-50.
18. Nirmalan PK, Katz J, Robin AL, Tielsch JM, Namperumalsamy P, Kim R, Narendran V, Ramakrishnan R, Krishnadas R, Thulasiraj RD, Suan E. Prevalence of vitreoretinal disorders in a rural population of southern India: the Aravind Comprehensive Eye Study. Arch Ophthalmol 2004; 122 (4): 581-6.
19. Krishnan T, Ravindran RD, Murthy GVS, Vashist P, Fitzpatrick KE, Thulasiraj RD, John N, Maraini G, Camparini M, Chakravarthy U, Fletcher AE. Prevalence of early and late Age-Related Macular Degeneration in India: The INDEYE Study. Invest Ophthalmol Vis Sci 2010; 51: 701-707
20. Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology 2007; 114 (2): 253-62.
21. Wang JJ, Rochtchina E, Lee AJ, Chia EM, Smith W, Cumming RG, Mitchell P. Ten-year incidence and progression of age-related maculopathy: the blue Mountains Eye Study. Ophthalmology 2007; 114 (1): 92-8.
22. Mukesh BN, Dimitrov PN, Leikin S, Wang JJ, Mitchell P, McCarty CA, Taylor HR. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology 2004; 111 (6): 1176-82.
23. Jonasson F, Arnarsson A, Peto T, Sasaki H, Sasaki K, Bird AC. 5-year incidence of age-related maculopathy in the Reykjavik Eye Study. Ophthalmology 2005; 112 (1): 132-8.
24. Delcourt C, Lacroux A, Carrière I; POLA Study Group. The three-year incidence of age-related macular degeneration: the “Pathologies Oculaires Liées à l’Age” (POLA) prospective study. Am J Ophthalmol 2005; 140 (5): 924-6.
25. van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol 2003; 121 (4): 519-26.
26. Leske MC, Wu SY, Hennis A, Nemesure B, Yang L, Hyman L, Schachat AP; Barbados Eye Studies Group. Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology 2006; 113 (1): 29-35.
27. Miyazaki M, Kiyohara Y, Yoshida A, Iida M, Nose Y, Ishibashi T. The 5-year incidence and risk factors for age-related maculopathy in a general Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci 2005; 46 (6): 1907-10.
28. Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology 1999; 106 (6): 1049-55.
29. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992; 99 (6): 933-43.
30. Bressler SB, Muñoz B, Solomon SD, West SK; Salisbury Eye Evaluation (SEE) Study Team. Racial differences in the prevalence of age-related macular degeneration: the Salisbury Eye Evaluation (SEE) Project. Arch Ophthalmol 2008; 126 (2): 241-5.
31. Klein R, Clegg L, Cooper LS, Hubbard LD, Klein BE, King WN, Folsom AR. Prevalence of age-related maculopathy in the Atherosclerosis Risk in Communities Study. Arch Ophthalmol 1999; 117 (9): 1203-10.
32. Klein R, Klein BE, Marino EK, Kuller LH, Furberg C, Burke GL, Hubbard LD. Early age-related maculopathy in the cardiovascular health study. Ophthalmology 2003; 110 (1): 25-33.
33. Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 1995; 102 (10): 1450-60..
34. VanNewkirk MR, Nanjan MB, Wang JJ, Mitchell P, Taylor HR, McCarty CA. The prevalence of age-related maculopathy: the visual impairment project. Ophthalmology 2000; 107 (8): 1593-600.
35. Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, de Jong PT. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 1995; 102 (2): 205-10.
36. Delcourt C, Diaz JL, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration. The POLA Study. Pathologies Oculaires Liées à l’Age. Arch Ophthalmol 1998; 116 (8): 1031-5.
37. Topouzis F, Coleman AL, Harris A, Anastasopoulos E, Yu F, Koskosas A, Pappas T, Mavroudis L, Wilson MR. Prevalence of age-related macular degeneration in Greece: the Thessaloniki Eye Study. Am J Ophthalmol 2006; 142 (6): 1076-9. 5.
38. Krishnaiah S, Das T, Nirmalan PK, Nutheti R, Shamanna BR, Rao GN, Thomas R. Risk factors for age-related macular degeneration: findings from the Andhra Pradesh eye disease study in South India. Invest Ophthalmol Vis Sci 2005; 46 (12): 4442-9.