Geographic Atrophy
Geographic Atrophy
Authors:
Fernanda Vaz, MD
Ophthalmology Department, Centro Hospitalar de Lisboa Ocidental, Lisbon, Portugal
Maria Picoto, MD
Ophthalmology Department, Hospital Beatriz Ângelo, Lisbon, Portugal
Updated/reviewed by the authors, July 2017.
Introduction
Geographic atrophy (GA) is considered the late stage of the dry form of age-related macular degeneration (AMD)(1).
GA is less common than neovascular AMD and it is responsible for 10-20% of cases of legal blindness in this condition(2,3,4), affecting more than 5 million people wordwilde(5).
Currently there is no approved or effective treatment to prevent either the onset or progression of GA, however, in recent years, significant progress has been made in understanding the pathogenesis of GA, which has led to a number of new potential therapies currently undergoing clinical trial evaluation(6,7,8).
Definition
Usually defined as any sharply delineated round or oval area of hypopigmentation, or apparent absence of the retinal pigment epithelium (RPE), in which choroidal vessels are more visible than in surrounding areas, that must be at least 175 μm in diameter (Figure 1)(1).
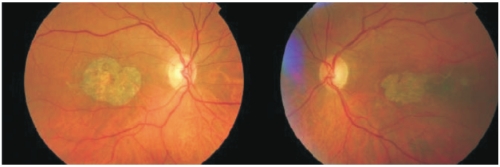
Figure 1. Fundus photography showing a sharply delineated area of hypopigmentation or apparent absence of RPE in both eyes of the same patient.
However larger dimensions have also been proposed for GA minimum limit. The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) specified a diameter ≥ 250 μm for definition of GA and in the AREDS2 study the minimum diameter of the area was increased to 433 μm. Currently, there is no international consensus on the minimum diameter for the diagnosis of GA(8).
In addition, changes have been proposed in the grading of AMD severity level regarding the presence of GA. In the AREDS grading system, the presence of GA qualifies patients as having either intermediate or advanced AMD, depending on whether or not there is central involvement(9).
In the classification system recently developed by the Beckman Initiative for Macular Research, the presence of any GA regardless of location qualifies as evidence of advanced AMD(10).So far there is no approved or effective treatment to prevent the onset and progression of GA
One eye with GA and choroidal new vessels (CNV) is considered as having exudative form(11).
Epidemiology and risk factors
The global prevalence of GA is 0.66% in all ages but it occurs in 0.34% between 65-74 years old, 1.3% between 75-84 and 4.4% over 85 years old.
The prevalence increases to 22% after 90 years of age(2).
It corresponds to half of exudative form prevalence(4,12-14).
A recent meta-analysis based on data in the United States reported that the incidences of GA and neovascular AMD were 1.9 and 1.8 per 1,000 American whites aged ≥ 50 years. Prevalence rates for the two forms of advanced AMD were comparable across all age groups(15).
For subjects with mild or intermediate AMD the 15-year cumulative incidence of neovascular AMD is approximately 2.0% and for progression to pure GA it is approximately 1.3%(16).
The most consistent risk factors are age, familiar history and tobacco smoking, like in other forms of AMD((2,4,13).
In AREDS, GA was also associated with: high BMI (body mass index); use of calcium channel blockers and ß-blockers; not using anti-acids; not using hormone replacement (woman); light iris color and less education level(14-17).
The prevalence of GA is lower in blacks than in whites (2.1% vs 4.8%) like in exudative forms(18,19).
In Rotterdam and Beaver Dam Eye Study, serum HDL cholesterol was directly associated with GA, however this association was not found in Blue Mountains Eye Studyl(20).
In this study, diabetes and the ratio total/HDL cholesterol were linked to increased risk of GA.
As complement system seems to play an essential role in this disease, the complement factor H (CFH) gene located at chromosome 1q32, and others as CFB, CFI, C2 and C3, have been implicated in the development of both forms of AMD(21-23).
Some studies have linked specifically 5p region and 4q 32 region with GAl(24,25).
Only recently, more detailed genome-wide association studies have investigated whether the 2 subtypes of advanced AMD segregate separately in families and associate with different disease variantsl(26).
The variants in the 10q26 locus confer increased risk for both advanced AMD subtypes, but imparts greater risk for CNV than for GA.
Other loci were detected with suggestive associations that differ for advanced AMD subtypes and deserve follow-up in additional studiesl(6).
Pathology
Accordingly to AREDS the most common sequence of events leading to GA is the progression of a large drusen to hyperpigmentation, followed by regression of the drusen, hypopigmentation and ultimately RPE cell death, with development of an atrophic area of retina and underlying choriocapillaris, sometimes preceded by the appearance of refractile deposits.
This evolution can be longer than 6 years(27-29).
The average time from entering the AREDS Study to initial appearance of GA has been shown to be approximately 5 to 6 years in the presence of confluent or large (> 125 μm) drusen and hyperpigmentation, as compared with 2.5 years in the presence of hypopigmentation(6).
The presence of large confluent drusen is a significant risk factor for developing GA(30).
Drusen were found in 100% of patients at the site of later GA development; drusen > 125 μm, confluent drusen and hyperpigmentation were present in > 90% of eyes, whereas drusen > 250 μm and hypopigmentation were present in > 80% eyes(31).
Less frequently, GA can follow a drusenoid RPE detachment, regression of a CNV membrane or a RPE rupture(11,32,33).
Higher prevalence of reticular pseudodrusen (RPD) has been reported in patients with GA compared with early or intermediate AMD. Also, the presence of RPD appear to represent a meaningful risk factor associated with progression to GA(34,36).
In some eyes, atrophy was related to a micro reticular pigment pattern distributed around the perimeter of the fovea(33).
Most histopathologic studies suggest that RPE cells are the primary target in GA and its death results in choriocapillaris atrophy(37,38).
Autofluorescent pigments as lipofuscin that accumulate in RPE cells, contribute to a decline of the cell function and degeneration conducting to GA(39).
Although RPE cells are the most involved, the outer nuclear layer is also severely affected with dysfunction and death of photoreceptors. Probably rods are the first affected photoreceptors(40,41).
The mechanisms of RPE death are best studied at junctional zone.
Here, lipofuscin may occupy 30% of RPE cell and may interfere with its metabolism, conducting to death.
These mechanisms include oxidative stress and inflammation(42-45).
Macrophages are often seen in areas of GA, apparently phagocytosing pigment and debris resulting of normal cells deletion(46).
Diagnosis
GA can be distinguished from other forms of dry AMD based on stereo biomicroscopy and color fundus photography(6).
The definition of GA is based on clinicohistopathologic investigations that have shown that clinically visible areas of atrophy are characterized by cell death in the RPE, outer neurosensory retina and choriocapillaris.
With modern in vivo imaging technology, these findings can be confirmed(6).
Spectral-domain optical coherence tomography (SD-OCT) and fundus autofluorescence (FAF) allow for noninvasive and rapid quantitative morphological assessment of GA in the clinical setting(6).
Fundus
Fundoscopy in GA typically shows a well-circumscribed oval or round area of pigment epithelium atrophy with sharply demarcated borders and increased visibility of choroidal vessels, usually sparing the fovea until late stages (Figure 2).
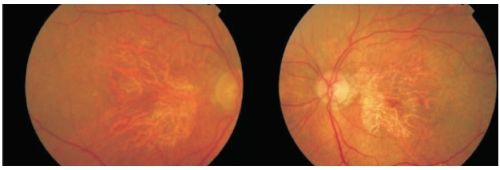
Figure 2. Fundus photography showing a well-circumscribed round area of pigment epithelium atrophy, sparing the fovea in right and left eyes.
All precursor lesions of this final appearance can also be present: large drusen (>125 microns), focal pigmentation changes and refractile deposit(27,28).
Angiography
On fluorescein angiography, GA appears as a sharply delineated window defect due to atrophy of overlying layers of RPE (Figure 3).
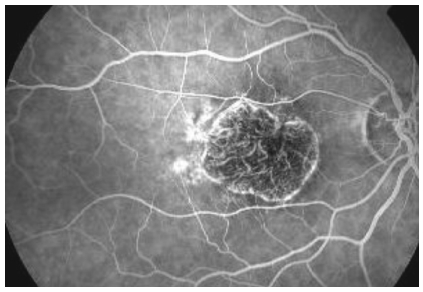
Figure 3. Fluorescein angiography showing a sharply delineated window defect.
A prolonged choroid filling phase has been described as a clinical marker for changes in Bruch’s membrane and as a risk factor for development of GA(29).
Despite these aspects in GA, fluorescein angiography may be indicated only in atypical cases, in order to allow the correct diagnosis(47).
Optical coherence tomography (OCT)
OCT scan shows thinning of hyperreflective external band, corresponding to attenuation of RPE/Bruch’s complex, and deeper hyperreflectivity because of loss of outer layers including photoreceptors (Figure 4)(48,49).
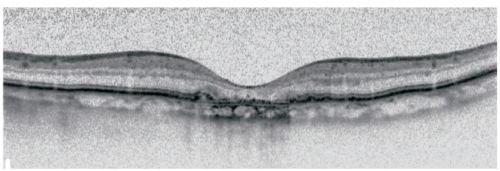
Figure 4. Spectralis OCT: Thinning of hyperreflective external band (because of attenuation of RPE/Bruch’s complex) and deeper hyperreflectivity.
In high resolution OCT the atrophic area shows hyperreflective clumps at different levels, segmented plaques of the outer band and elevations with variable reflectivity(41).. In the perilesional area there are elevations of the outer retinal layers, as well as thickening of outer hyperreflective band. At the junction area the outer band shows different degrees of loss(50).
Recently, changes in OCT that precede the development of GA have been identified. These changes include the presence of hyperreflective foci in the retina overlying drusen, a subsidence of the inner nuclear layer (INL) and outer plexiform layer (OPL) with the development of hyporeflective, wedge-shaped bands, and increased signal transmission below the level of the RPE.
These anatomic changes might be used to identify patients early in the course of GA development who might still be at a reversible stage and therefore amenable to intervention(51-53).
OCT imaging may also contribute to a better understanding of the underlying pathologic mechanisms in AMD and GA, may suggest new biomarkers related to disease progression, and might potentially indicate new therapeutic targets in AMD(8).
Fundus autofluorescence (FAF)
FAF is the current standard imaging technology for the morphological assessment of GA(8).
Fundus spectrophotometric studies in vivo by Delori and co-workers have shown that FAF represents an accumulation of lipofuscin in the lysossomes of RPE cells, mainly derived from photoreceptors outer segments degradation.
The compound is found as micrometer-sized spherical particles and is characterized by yellow autofluorescence when exposed to blue light(54-56).
It has been shown with confocal scanning laser ophthalmoscopy (cSLO) that FAF response is very low or extinguished in areas of atrophy.
The lack of RPE cells or its low number and therefore of lipofuscin, (the dominant fluorophore) explain this reduction(57).
Increased FAF precedes development of GA(58,59).
FAF is increased in junctional zone around areas of atrophy, and intensity seems to correlate with extension of the atrophic area and also with reduction of retinal sensitivity detected by fundus perimetry (Figure 5)(60,61).
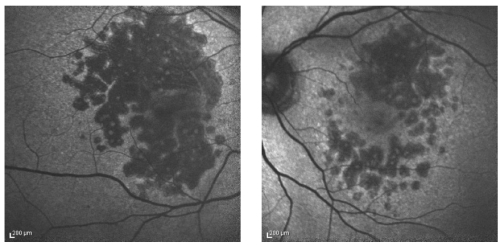
Figure 5. FAF showing increased fluorescence in the junctional zone around areas of atrophy.
The FAM-Study Group developed the classification of abnormal FAF according to distinct patterns in the junctional zone of the GA area and identified the following five primary phenotypes based on the presence of increased hyper-autofluorescence: None, Focal, Banded, Patchy and Diffuse(62).
Holz et al. showed that the phenotypic features of FAF abnormalities had a much stronger impact on atrophy progression than any other risk factor that has been addressed in previous studies on progression of GA attributable to AMD, and introduced the ‘diffuse trickling' pattern that is associated with an extremely rapid progression of atrophy(63).
These hyperfluorescent areas in the junctional zone are believed to represent regions of cells that are stressed and more likely to become atrophic(8).
Psychophysical Tests of Visual Function
GA develops gradually with the formation of scotomas that spare the fovea initially, expanding into the central visual field late in the course of disease(8).
The most common assessment of visual function, best corrected visual acuity (BCVA), usually fails to reveal the functional deficits experienced by patients with foveal-sparing GA(8).
Functional assessments beyond BCVA including multifocal electroretinography (mfERG), microperimetry, low-luminance visual acuity (LLVA), reading speed and contrast sensitivity, should be used to more fully assess visual impairment(8).
Clinical evolution
Eyes with GA may also develop CNV. In one study, 7% of eyes with GA developed CNV in 2 years.
The strongest risk factor for developing CNV in one eye with GA is the presence of CNV in the fellow eye(64).
The 4-year rate of developing CNV is 11% if the other eye has pure GA but increases to 34% if there is CNV.
As other forms of late AMD, GA tends to be bilateral (over 50% of cases) and there is high symmetry between eyes for total atrophic area, presence of peripapillary atrophy and enlargement rate((64-66).
On the other hand there is a high interindividual variability(67).
Longitudinal studies have shown that progression rates vary widely among patients(10).
The mean overall enlargement rate of atrophic area is 2.6 mm2/year.
Eyes with larger areas of atrophy at baseline tend to have larger enlargement rates(65).
The Beaver Dam Eye Study showed that eyes with multifocal disease had larger increase in area of GA and progressed to foveal involvement more frequently than eyes with single foci of disease over 5 years (1.2 vs 2.24 mm2)(68).
GA often first develops surrounding the fovea, sparing the central area. Because of that, the correlation between visual acuity and area of atrophy can be complex(69-71).
The loss of three lines (ETDRS scale) was observed by Suness et al. in 31% of studied eyes by 2 years and 53% by 4 years. More than half of patients with GA encroaching upon the fovea will suffer this severity of vision loss in the same time frame when compared with extrafoveal GA(71).
Even when GA is not center involving, patients can suffer significant deficits in visual function, such as compromised reading ability and impaired vision in dim lighting(72).
The occurrence of GA with concomitant CNV is associated with an even higher risk of severe vision than GA alone(73-75).
Suness et al. showed that 86% of eyes with GA that developed CNV lost 3 or more lines of visual acuity over 2 years compared with only 27% in eyes with GA that did not develop CNV(64).
The risk of visual acuity loss was higher in eyes with better visual acuity at baseline and with lightest iris color(71).
Usually vision loss is bilateral because of lesion symmetry, and evolution since first signs to legal blindness is quite variable(65,66).
Because of rods paucity, reduction of foveal cone function and also because of switching from central to eccentric fixation, other visual functions as the contrast sensitivity, reading rate and dark adaptation are decreased in GA(69,72,72).
The low luminance visual dysfunction and the reduction of the maximum reading rate seem to be significant risk factors for subsequent visual acuity loss(69).
Management
Prevention
In AREDS, dietary supplements as zinc, anti-oxidants, vitamins C and E and beta-carotene have been shown to reduce the risk of progression in participants in categories 3 and 4 to advanced AMD (25% in 5 years), however, in the group with GA away from the center (category 3), this reduction was not statistically significant.
Despite of that the AREDS Report nº 8 concluded that those with noncentral GA also should consider taking a supplement of antioxidants plus zinc(9,17).
Macular xantophylls and polyunsaturated fatty acids seem to be associated with a lower risk of advanced AMD(76,77).
Because of that antioxidant effect of macular pigments, lutein, zeaxanthin and omega-3 fatty acids have been tested in the AREDS 2 study(76,78).
The researchers also tried substituting lutein and zeaxanthin for beta-carotene, which prior studies had associated with an increased risk of lung cancer in smokers.
The AREDS 2 study found that while omega-3 fatty acids had no effect on the formulation, lutein and zeaxanthin together appeared to be a safe and effective alternative to beta-carotene.
Subgroup analysis indicated that those with the lowest intake of lutein and zeaxanthin, supplemental lutein and zeaxanthin were protective(79).
Low dietary glycemic index also seems to reduce the risk of evolution to advanced AMD(79).
Other behavioral factors such as stop smoking, as it represents a key modifiable risk factor and control of BMI may play an important role on prevention(80-82).
New treatments
So far there is no approved or effective treatment to prevent the onset and progression of GA(6,30).
Although the main target for GA remains unknown, several trials are investigating strategies regarding these pathways and therapeutic targets:
- Visual cycle toxic products (visual cycle modulators);
- Inflammation, complement, and ECM (mTOR inhibitors, complement inhibitors, MMP inhibitors);
- Lipoprotein accumulation (LDL lowering drugs);
- Beta-amyloid accumulation (anti-amyloid beta);
- Oxidative stress (antioxidants and neuroprotectant);
- Choriocapillaris atrophy (choroidal perfusion enhancers);
- RPE and photoreceptor loss (Stem cell therapy and neurotrophins);(6)
Visual cycle Inhibitors
Accumulation of lipofuscin and melanolipofuscin granules have been observed at the sites of RPE atrophy in GA and associated with GA pathogenesis(7).
ALK-001 is a modified vitamin A molecule, which slows the formation of lipofuscin and RPE apoptosis.
This compound was initially designed to treat Stargardt’s disease. It may also benefit GA by reducing toxic A2E, all-trans retinal, and lipofuscin accumulation.
A phase 1 study was designed to assess the safety and pharmacokinetics of oral ALK-001 capsules in 40 healthy volunteers with no results posted yet(6,7).
Fenretinide and Emixustat (ACU-4429) were also studied but there was no evidence of benefit(6,7,83-85).
Anti-inflammatory Agents
Chronic inflammation is believed to play an important role in AMD pathogenesis.
Copaxone (or Glatiramer Acetate) functions to induce suppressor T cells and down-regulates inflammatory cytokines.
A phase I trial was designed to test its safety as well as its efficacy in the prevention of GA progression or conversion of dry AMD to neovascular AMD.
The participant recruitment was suspended with no results posted yet(7).
Lampalizumab is an intravitreal (IVT) administered recombinant monoclonal antibody fragment directed against complement factor D in the alternative complement pathway.
In phase 2 trial (MAHALO study) monthly Lampalizumab showed 20.4% reduction rate in GA area at 18 months in patients with this form of dry AMD compared with monthly sham treatment(82).
Lampalizumab is currently being evaluated in a large, multicenter, phase 3 clinical trial for GA.
Primary outcomes will measure change in GA area after 48 weeks and BCVA up to 2 years after beginning the study(7,30,83).
LFG 316 is an antibody against the C5 portion of the complement pathway administered IVT(6).
The phase 2 trial designed to test the safety and efficacy of lower and higher doses of IVT LFG316 using 18 successive monthly injections in patients with GA was completed, but the results have not yet been published as reported on ClinicalTrials.gov.
APL2 is a derivative of compstatin, a small peptide inhibitor of complement factor C3, that is currently in phase 2 trial (FILLY) to assess the safety, tolerability and evidence of activity of multiple IVT injections of APL-2 in subjects with GA as reported on ClinicalTrials.gov.
Zimura (ARC-1905) is a chemically synthesized aptamer that inhibits complement factor C5.
Phase I trial for Other inflammation suppressor is fluocinolone acetonide, a glucocorticoid used as an intravitreal implant.
Although currently there is no drug proven effective, in the next decade some of the research lines will probably be able to find a more effective treatment for the atrophic form of AMD.
Dry AMD evaluated the safety and tolerability of IVT Zimura injection in patients with GA. The study was completed with no results posted, but Ophthotech plans to initiate Phase II/III clinical trial(7).
Flucinolone acetonide, Eculizumab and Sirolimus (Rapamicina) were also studied, but phase 2 trials failed to demonstrate efficacy(6,7,30).
Lipid Modulators
Lipids are also found in drusen and several studies demonstrate that lipids accumulate at the site of subsequent formation of AMD depositsl(86).
Barbosa et al. showed that statin use had a statistically significant effect in reducing AMD incidence in participants aged 68 years and olderl(87).
However the association of statin intake and AMD remains controversial as demonstrated by a 2012 Cochrane database review and to date no trials in GA with this target have been identifiedl(88).
Amyloid
The rationale for this new treatment strategy for AMD is related to the presence of amyloid β in drusen.
GSK933776 is a humanized monoclonal antibody intended to modulate amyloid-β levels in patients with GA secondary to AMD administered via intravenous infusion(6,7).
A phase 2 study is completed, however the study results have not been posted (ClinicalTrials.gov).
RN6G is a humanized monoclonal antibody intended to prevent accumulation of amyloid β-40 and β-42 and preserve the photoreceptors and the RPE delivered by intravenous injections(6,7).
A phase 2 study has terminated, but has not been successful, as reported on ClinicalTrials.gov.
MRZ-99030 is a dipeptide containing d-tryptophan and 2-amino-2-methylpropionic acid in clinical development for the topical treatment (eye drops) of glaucoma and AMD(7,89).
Neuroprotection
Neuroprotectants have been shown to protect photoreceptors in in vitro models of retinal degeneration and animal models of glaucoma and retinitis pigmentosa.
The mechanism by which neurotrophic factors, such as ciliary neurotrophic factor (CNTF) and basic fibroblast growth factor, protect photoreceptors is not fully understood, and it should be noted that the relevance of these animal models in AMD is not clear.
The RPE and retina both produce fibroblast growth factor and the retina produces CNTF(6).
Brimonidine stimulates the production of neurotrophic factors and can protect photoreceptor cells in animal models of retinal degeneration; it has been formulated as an intravitreal implant which delivers the drug to the retina over a 3-month period.
A Safety and Efficacy Study of Brimonidine Intravitreal Implant in Geographic Atrophy Secondary to Age-related Macular Degeneration (BEACON) (phase 2) is ongoing, with progression of the area of GA at month 12 being the primary endpoint (clinicalTrials.gov)(6).
According to the preliminary results launched at the AAO (American Academy og Ophthalmology) 2016 annual meeting, Brimonidine Intravitreal Implant (22-gauge polymer similar to the one used in ozurdex®, one injection twice a year) caused a significant slowing of disease progression with no adverse effects.
Allergan has completed enrollment of a follow-up study aiming to develop a more potent delivery method(90).
UF-021 (isopropyl unoprostone) is a prostaglandin analog that was investigated in Japan in a phase 2 clinical study in patients with mid- to late-stage retinitis pigmentosa(6).
A phase 2 trial for dry AMD in United States is completed but the study results have not been published yet (ClinicalTrials.gov).
A sustained-release platform with encapsulated human RPE cells engineered to release CNTF has been developed and was tested in a phase 2 study.
However, no benefit in the progression of lesion expansion was found(6,91).
Tandospirone (AL-8309B) a selective serotonin 1A agonist that protects the retina from light damage was investigated, but the phase 3 trial (GATE study) was discontinued in 2012 owing to lack of efficacy(6).
Oxidative stress protection
The AREDS trial has reported that daily high doses of antioxidants β-carotene, vitamins C and E and zinc reduced progression to advanced AMD by 25%.
However, the 25% benefit was reported only for the progression to neovascular AMD; there was no benefit in the progression to central GA. Furthermore, the AREDS antioxidant mineral formulation was reported as having no significant effect on the progression of GA in a more recent study(92).
The AREDS2 trial assessed the addition of lutein, zeaxanthin, and/or long-chain ω-3 fatty acids to the original AREDS formulation on progression to advanced AMD.
The study completed in early 2013 found that omega-3 fatty acids had no overall additional effect on the formulation, in a median follow-up period of 5 years(7,93).
Analyses of the AREDS participants over a 12-year period found that participants with the highest omega-3 fatty acids intake were 30% less likely than their peers to develop central GA and neovascular AMD(93).
Several studies are ongoing investigating the effects of ω-3 fatty acids, the ratio of omega-3 to omega-6 fatty acids, and lutein and zeaxanthin (AREDS2 study) in patients with AMD(6,94,95).
OT-551, a small molecule with antioxidant and antinflammatory properties was investigated, however phase 2 study results showed no significant effect on lesion enlargement, retinal sensitivity or total drusen area(96).
Choroidal Perfusion Enhancers
Choroidal circulation provides the nutrition and removes the waste from the retina/RPE.
As a consequence of a reduced choroidal blood flow, metabolic wastes are accumulated in photoreceptor cells, Bruch’s membrane and RPE cells.
Those events can lead to development of GA.
Therefore, improving choroidal blood flow could facilitate the removal of metabolic wastes from RPE, Bruch’ membrane and photoreceptor cells to halt AMD disease progression(7)..
MC-1101 is a topical agent with anti-inflammatory and antioxidative properties shown to increase choroidal blood flow; its intended action is to prevent rupture of Bruch’s membrane.
Phase Ib clinical trial showed that topical instillation of 1% MC-1101 produced no significant cardiovascular or ocular toxicity.
A phase II/III is ongoing, 60 patients will receive topical 1% ophthalmic solution, and be assessed for visual function over 24 months(6,7).
Trimetazidine was also studied but failed to prevent progression of GA(6).
Stem cells
Replenishing the lost or degenerating RPE cells in GA before the photoreceptors are irrevocably damaged with stem cell-derived RPE cells represents the forefront in the practice of regenerative medicine(7).
RPE can be differentiated from human embryonic stem cells (hESCs) or human induced pluripotent stem cells (iPSCs).
Both display RPE-like morphology and express typical RPE markers and have the ability to phagocytose photoreceptor segments.
When they were transplanted subretinally into rat or mouse model of RPE insufficiency, the grafted cells were retained and retinal function improved(7).
Two prospective phase 1/2 studies were done to assess safety and tolerability of subretinal transplantation of hESC-derived RPE in nine patients with Stargardt's macular dystrophy and nine with atrophic AMD.
Transplanted patients were followed up for a median of 22 months.
There was no evidence of adverse proliferation, rejection, or serious ocular or systemic safety issues related to the transplanted tissue.
BCVA, monitored as part of the safety protocol, improved in ten eyes, improved or remained the same in seven eyes, and decreased by more than ten letters in one eye, whereas the untreated fellow eyes did not show similar improvements in visual acuity.
Vision-related quality-of-life measures increased for general and peripheral vision, and near and distance activities in both diseases.
The results of this study provide the first evidence of the medium-term to long-term safety, graft survival, and possible biological activity of pluripotent stem cell progeny in individuals with any disease.
The results suggest that hESC-derived cells could provide a potentially safe new source of cells for the treatment of various unmet medical disorders requiring tissue repair or replacement(97).
There are currently 2 other clinical trials (phase 1/2) using hESC-RPE cells (MA09-hRPE).
Both are designed to evaluate safety and tolerability of subretinal injection or transplantation of MA09-RPE cells in patients with dry AMD.
Secondary outcomes will measure the mean change of BCVA, autofluorescence photography and reading speed(6,7).
In another study, clonogenic human central nervous system stem cells (HuCNS-SC) will be evaluated for treatment of dry AMD.
Phase I/II study will investigate the safety and preliminary efficacy of unilateral subretinal transplantation of HuCNS-SC cells in subjects with GA secondary to AMD(6,7).
Conclusion
GA is a devastating blinding disease without any approved or effective treatment currently available.
Numerous clinical trials are ongoing with the purpose of finding a viable solution to prevent or treat the disease.
The AREDS trials show that AREDS formulation reduces the risk of AMD progression by 25%, while transplanted hESC-derived RPE cells show long-term safety, graft survival and possible biological activity shown by improved visual acuity in GA patients(6,7,30).
Future studies should focus on understanding the pathogenesis of the disease, which remains unclear.
Moreover, the development of advanced imaging system will provide state-of-art tools for analyzing GA pathophysiology and testing new therapeutics(7).
References - Geographic Atrophy
References - Geographic Atrophy
- Bird AC, Bressler NM, Bressler SB, et al. An International Classification and Grading System for Age-Related Maculopathy and Age-Related Macular Degeneration. The International ARM Epidemiology Study Group. Surv Ophthalmol. 1995;39:367-374.
- Buch H, Vinding T, Nielsen NV, et al. 14-year incidence progression and visual morbidity of age-related maculopaty: The Copenhagen City Eye Study. Ophthalmology. 2005;112:787-798.
- Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640-1642.
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933-943.
- Rudnicka AR, Jarrar Z, Wormald R, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580.
- Holz FG, Strauss EC, Schmitz-Valckenberg S, et al. Geographic Atrophy Clinical Features and Potential Therapeutic Approaches. Ophthalmology. 2014;121:1079-1091.
- Hanus J, Zhao F, Wang S. Current Therapeutic Development for Atrophic Age-related Macular Degeneration. Br J Ophthalmol. 2016;100(6):744.
- Srinivas S, Chakravarthy U, et al. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina. 2016;36:1806–1822.
- Age-Related Eye Disease Study Research Group. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in Age-Related Eye disease Study: AREDS report nº 8. Arch Ophthalmol. 2008; 119:1417-1436.
- Ferris FL III, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851.
- Gass JDM. Drusen and disciform macular detachment and degeneration. Arch Ophthalmol. 1973; 90:206-217.
- Vingerling JR, Dielmans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205-210.
- Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697-704.
- Fraser-Bell S, Wu J, Klein R, et al. Smoking, Alcohol Intake, Estrogen Use, and Age-related Macular Degeneration in Latinos: The Los Angeles Latino Eye Study. Am J Ophthalmol. 2006;141:79-87.
- Rudnicka AR, Kapetanakis VV, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85–93 e83.
- Klein R, Klein BE, Knudtson MD, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253-262.
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration: A case-control study in the Age-Related Eye Disease Study: AREDS report nº3. Ophthalmolgy.
- 2000;107:2224-2232.
- Friedman DS, Katz J, Bressler NM, et al. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology. 1999;106:1049-1055.
- Klein R, Klein BE, Knudtson M, et al. Prevalence of Age-related Macular Degeneration in 4 Racial/Ethnic Groups in the Multi-ethnic Study of Atherosclerosis. Ophthalmology. 2006;113:373-380.
- Klein R, Klein BE, Tomany SC. The Association of Cardiovascular Disease with the Long-term Incidence of Age-Related Maculopathy: The Beaver Dam Eye Study. Ophthalmology. 2002;110:1273-1280.
- Postel EA, Agarwal A, Caldwell J, et al. Complement Factor H Increases Risk for Atrophic Age-Related Macular Degeneration. Ophthalmology. 2006;113:1504-1507.
- Dominiek DG, Cornelia MD, Oostra BA, et al. Complement Component C3 and Risk of Age-Related Macular Degeneration. Ophthalmology. 2009;115:474-480.
- Cameron DJ, Yang Z, et al. HTRA1 variant confer similar risk to Geographic Atrophy and Neovascular Age-Related Macular Degeneration. Cell Cycle. 2007;6(9):1122-1125.
- MajewskiJ, Schultz DW, Weleber RG, et al. Age-Related Macular Degeneration: a genome scan in extended families. Am J Hum Genet. 2003;73:540-550.
- Abecasis GR, Yashar BM, Zhao Y, et al. Age-related macular degeneration: a high resolution genome scan for susceptibility loci in a population enriched for late-stage disease. Am J Hum Genet. 2004;74:482-494.
- Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol. 1997;123:199–206.
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study Severity Scale for Age-Related Macular Degeneration: Age-Related Eye Disease Study (AREDS) report nº 17. Arch Ophthalmol. 2005;123:1484-1498.
- Klein ML, Ferris FLIII, Armstrong J, et al. (AREDS Research Group). Retinal Precursors and the Development of Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmology. 2008;115:1026-1031.
- Pauleikhoff D, Spital G, Bird AC. A Fluorescein and Indocyanine Green Angiographic Study of Choriocapillaris in Age-related Macular Disease. Arch Ophthalmol. 1999;117:1353-1358.
- Kashani AH. Stem Cell Therapy in Nonneovascular Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFm1-9.
- Klein ML, Ferris FL 3rd, Armstrong J. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(6):1026-31.
- Age-Related Eye Disease Study Research Group. Natural History of Drusenoid Pigment Epithelial Detachment in Age-Related Macular Degeneration: AREDS report nº 28. Ophthalmology. 2010;117:489-499.
- Casswell AG Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985;69:397-403.
- Finger RP, Wu Z, Luu CD, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121:1252–1256.
- Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmology Vis Sci.
2013;54:7362–7369.
- Kovach JL, Schwartz SG, Agarwal A, et al. The relationship between reticular pseudodrusen and severity of AMD. Ophthalmology. 2016;123:921–9236.
- Lutty G, Grunwald J, Majji AB,et al. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35.
- McLeod DS, Taomoto M, Otsuji T, et al. Quantifying Changes in RPE and Choroidal Vasculature in Eyes with Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2002;43:1986-1993.
- Sparrow JR, Boulton M. Lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595-606.
- Curcio CA, Medeiros NE, Milican CL. Photoreceptor Loss in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236-1249.
- Kim SY, Sadda S, Humayun MS, et al. Morphometric Analysis of the Macula in Eyes With Geographic Atrophy due to Age-Related Macular Degeneration. Retina. 2002;22:464-470.
- Holz FG, Schutt F, Kopitz J, et al. Inhibition of Lysosomal Degradative Functions in RPE Cells by a Retinoid Component of Lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737-743.
- Beatty S, Koh H, Phil M, et al. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv Ophthalmol. 2000;45:115-134.
- Rózanowska M, Korytowski W, Rózanowski B, et al. Photoreactivity of Aged Human RPE Melanosomes: A Comparison with Lipofuscin. Invest Ophthalmol Vis Sci. 2002;43:2088-2096.
- Anderson DH, Mullins RF, Hageman GS, et al. A Role for Local Inflammation in the Formation of Drusen in the Aging Eye. Am J Ophthalmol. 2002;134:411-431.
- Coleman H R, Ferris III FL, Chew EY, et al. Age related macular degeneration. Lancet. 2008;372 (9652):1835-1845.
- Bischoff P, Speiser P. The role of angiography in age-related macular degeneration. Klin Monatsbl Augenheilkd. 1997;210:296-8.
- Shuman JS, Puliafito CA, Fujimoto JG, et al. Optical Coherence Tomography of Ocular Diseases. Second edition. Slack, 2004.
- Bearelly S, Chau FY, Koreishi A, et al. Spectral Domain Coherence Tomography Imaging of Geographic Atrophy Margins. Ophthalmology. 2009;116:1762-1769.
- Fleckenstein M, Issa PC, Holz FG, et al. High-Resolution Spectral Domain-OCT Imaging in Geographic Atrophy Associated with Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2008;49(9):4137-4144.
- Karl G Csaky. What Should We Be Measuring as a Geographic Atrophy Endpoint? AAO Subspeciality Day Retina Sillabus.2015; p154-156.
- Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography- defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121:2415-2422.
- Ouyang Y, Heussen FM, Hariri A, et al. Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology. 2013;120:2656-2665.
- Delori FC, Dorey CK, Staurenghi G, et al. In Vivo Fluorescence of the Ocular Fundus Exhibits Retinal Pigment Epithelium Lipofuscin Characteristics. Invest Ophthalmol Vis Sci. 1995;36:718-729.
- Sparrow JR, Fishkin N, Zhou J, et al. A2E, a Byoproduct of the Visual Cycle. Vision Res. 2003;43:2983-2990.
- Frangieh GT, Green WR, Fine SL. A histopathological Study of Best’s Macular Dystrophy. Arch Ophthalmol. 1982;100:1115-1121.
- Sparrow JR, Boulton M. Lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595-606.
- Von Ruckmann A, Fitzke FW, Bird AC. Fundus Autofluorescence in Age-Related Macular Disease Imaged with Laser scanning Ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38:478-86.
- Holz FG, Bellman C, Staudt S, et al. Fundus Autofluorescence and Development of Geographic Atrophy in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051-1056.
- Schmitz-Valkenberg S, Bültmann S, Dreyhaupy J, et al. Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:4470-4476.
- Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, et al. Fundus Autofluorescence and Fundus Perimetry in the Junctional Zone of Geographic Atrophy in Patients with Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2006;47:2648-2654.
- Bindewald A, Schmitz-Valckenberg S, Jorzik JJ et al. Classification of abnormal fundus autofluorescence patterns in the junctional zone of geographic atrophy in patients with age related macular degeneration. Br J Ophthalmol. 2005;89(7):874-8.
- Holz FG, Bindewald-Wittich A, Fleckenstein M et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463-72.
- Suness JS, Gonzalez-Baron J, Bressler NM, et al. The Development of Choroidal Neovascularization in Eyes with Geographic Atrophy Form of Age-Related Macular Degeneration. Ophthalmology. 1999;106:910-919.
- Suness JS, Margalit E, Bressler NM, et al. The Long-term Natural History of Geographic Atrophy from Age-Related Macular Degeneration: Enlargement of Atrophy and Implications for Interventional Clinical Trials. Ophthalmology. 2007;114:271-277.
- Schatz H, McDonald HR. Atrophic Macular Degeneration. Rate of Spread of Geographic Atrophy and Visual Loss. Ophthalmology. 1989;96:1541-1551.
- Bellmann C, Jorzik J, Spital G, et al. Symmetry of bilateral lesions in geographic atrophy in patients with age-related macular degeneration. Ophthalmology. 2002;120:579-584.
- Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am J Ophthalmol. 2008;146:692–699.
- Suness JS, Rubin GS, Broman A, et al. Low luminance Visual Dysfunction as a Predictor of Subsequent Visual Loss From Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmology. 2008;115:1480-1488.
- Maguire P, Vine AK. Geographic Atrophy of Retinal Pigment Epithelium. Am J Ophthalmol. 1986;102:621-625.
- Suness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of Atrophy and Visual Acuity Loss in the Geographic Atrophy Form of Age-Related Macular Degeneration. Ophthalmology. 1999;106:1768-1779.
- Suness JS, Applegate CA, Bressler NM, et al. Visual Function Abnormalities and Prognosis in Eyes with Age-Related Geographic Atrophy of the Macula and Good Visual Acuity. Ophthalmology. 1997;104(10):1677-1691.
- Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498.
- Schatz H, McDonald HR. Atrophic macular degeneration. Rate of spread of geographic atrophy and visual loss. Ophthalmology. 1989;96:1541–1551.
- Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115:741–747.
- Coleman H, Chew E. Nutritional Supplementation in Age-Related Macular Degeneration. Curr Opin Ophthalmol. 2007;18:220-223.
- Age-Related Eye Disease Study Research Group. Risk Factors for the Incidence of Advanced Age-Related Macular Degeneration in Age-Related Eye Disease Study: AREDS report nº 23. Arch Ophthalmol. 2008;126:1274-1279.
- Chiu CJ, Milton RC, Klein R et al. Dietary Compound Score and Risk of Age-Related Macular Degeneration in the Age-Related Eye Disease Study. Ophthalmology. 2008;116: 939-946.
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005-15.
- Khan JC, Thurlby DA, Shahid H, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75-80.
- Age-Related Eye Disease Study Research Group. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in Age-Related Eye Disease Study: AREDS report nº 19. Ophthalmology. 2005;112:533-539.
- Age-Related Macular Degeneration PPP - Updated 2015. AAO Retina/Vitreous PPP Panel, Hoskins Center for Quality Eye Care.
- Jack LS, Sadiq MA, Do DV,Nguyen QD. Emixustat and Lampalizumab: Potential Therapeutic Options for Geographic Atrophy. Dev Ophthalmol. 2016;55:302-9.
- Mata NL, Lichter JB, Vogel R, et al. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33:498–507.
- http://www.businesswire.com/news/home/20160525006550/en/Acucela-Announces-Top-Line-Results-Phase-2b3-Clinical
- Elisa Buschini, Antonio M Fea, Carlo A Lavia, et al. Recent developments in the management of dry age-related macular degeneration. Clin Ophthalmol. 2015;9:563–574.
- Barbosa DT, Mendes TS, Cíntron-Colon HR, et al. Age-related macular degeneration and protective effect of HMG Co-A reductase inhibitors (statins): results from the National Health and Nutrition Examination Survey 2005-2008. Eye (Lond). 2014;28(4):472-80.
- Gehlbach P, Li T, Hatef E. Statins for age-related macular degeneration. Cochrane Database Syst Rev. 2012;(3):CD006927.
- Parsons CG, Ruitenberg M, Freitag CE, et al. MRZ-99030 - A novel modulator of Aβ aggregation: I - Mechanism of action (MoA) underlying the potential neuroprotective treatment of Alzheimer's disease, glaucoma and age-related macular degeneration (AMD). Neuropharmacology. 2015;92:158-69.
- https://www.aao.org/interview/brimonidine-geographic-atrophy
- Zhang K, Hopkins JJ, Heier JS, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:6241–5.
- AREDS Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127:1168–74.
- Sangiovanni JP, Agron E, Meleth AD, et al. {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90(6):1601-7.
- Konstantin Petrukin. New Therapeutic Targets in Atrophic Age-Related Macular Degeneration. Expert Opin Ther Targets. 2007;11:625-639.
- Tanito M, Li F, Elliot MH, et al. Protective effect of TEMPOL derivates against Light-Induced Retinal Damage in Rats. Invest Ophthalmol Vis Sci. 2007;48:1900-1905.
- Wong WT, Kam W, Cunningham D, et al. Treatment of geographic atrophy by the topical administration of OT-551: results of a phase II clinical trial. Invest Ophthalmol Vis Sci. 2010;51(12):6131–6139.
- Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–516.