Diagnostic usefulness of indocyanine green angiography (ICGA) in age-related macular degeneration (AMD)
Diagnostic usefulness of indocyanine green angiography (ICGA) in age-related macular degeneration (AMD)
Authors:
Maribel Fernández, MD, PhD1,3,4
María Gil, MD1,3
Felipe Gonzalez2
Francisco Gómez-Ulla, MD, PhD1,3,4
1 Instituto Oftalmológico Gómez-Ulla. Santiago de Compostela. Spain.
2 Optometrist. Unit of Optometry. Instituto Oftalmológico Gómez-Ulla. Santiago de Compostela. Spain.
3 Department of Ophthalmology, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela. Spain.
4 University of Santiago de Compostela. Santiago de Compostela. Spain.
Updated/reviewed by the authors, July 2017.
Age-related macular degeneration (AMD), is a leading cause of severe central vision loss and legal blindness among people over 55 years of age in the industrialized countries (1-4), and thus represents a major worldwide sociosanitary problem.
Exudative (neovascular) AMD is a less common form of the disease, but is responsible for approximately 80-90% of all cases of severe vision loss (5-7). Choroidal neovascularization (CNV) in its different forms of presentation is the main cause of complications and vision loss in these patients.
The classification of CNV based on its angiographic characteristics was regarded as very important in the past, when treatment fundamentally consisted on thermal laser ablation or photodynamic therapy (PDT). However, at present, the development of spectral domain optical coherence tomography (SD-OCT) and the use of intravitreal injections of antiangiogenic drugs have led to a genuine revolution in the diagnosis, follow-up and prognosis of these patients.
Anti-vascular endothelial growth factor (VEGF) therapy is the treatment of choice in patients with exudative AMD, though there are cases where other treatments may be used alone or in combination in order to increase efficiency.
OCT has displaced other diagnostic techniques used for patient control in the frequent follow-up visits of the disease. Today, SD-OCT and noninvasive fundus autofluorescence imaging can provide additional anatomical information beyond the fluorescein angiography (FA), including visualization of the layers of the retina and choroid involved with the neovascular membrane. Moreover, swept source OCT (SS-OCT) is a new OCT technology, which have enabled a even faster acquisition and a reduction in the signal-to-noise ratio. The use of OCT has increased dramatically during the past decade, whereas the use of FA had declined considerably.
In addition, the more recent introduction of OCT angiography (OCTA) and the increasing experience in interpreting OCT images has reduced even further the need for FA and its associated side effects.
FA remains however, in our opinion, a necessary technique at the time of diagnosis, since it affords prognostic information, and is useful for re-evaluating patients with a poor response to treatment. In fact, the American Academy of Ophthalmology AMD Preferred Practice Pattern advocates the use of intravenous fundus FA when a patient complains of a new unexplained blurred vision and it is also indicated in the following situations: to detect the presence and determine the extent, type, size, and location of CNV; and, to detect persistent or recurrent CNV following treatment(9).
Nowadays, the retina and imaging community is excited to see if improvements in OCT technology and the introduction of the OCTA could eliminate the FA(9).
Indocyanine green angiography (ICGA) is useful for the study of occult CNV (OCNV), and particularly in identifying the characteristic patterns of idiopathic polypoidal choroidal vasculopathy (IPCV) and retinal angiomatous proliferation (RAP). It is important to correctly diagnose these disorders, since they respond differently to treatment. The main advantage of ICGA is that it allows specific analysis of choroidal circulation and its interactions with the retina. However, interpretation of the ICGA images is complex, and in some cases requires contrasting with the data obtained by means of other techniques such as FA or OCT, and with correct biomicroscopic assessment of the macula.
The present chapter analyses the information that we can obtain from ICGA in application to the diagnosis and follow-up of AMD, and defines the current most precise indications of the technique, and its usefulness in daily clinical practice.
Thus, an analysis is made of the characteristic ICGA images found in patients with AMD, the precise indications of the technique, and the situations in which ICGA proves essential for establishing a correct diagnosis to ensure adequate treatment of this complex disease.
INDOCYANINE GREEN ANGIOGRAPHY (ICGA)
Indocyanine green (ICG) is a tricarbocyanine dye with both hydrophilic and lipophilic properties(10). The empirical formula of ICG is C43H47N2NaO6S2. The molecular weight is 775 Daltons. When dissolved in saline solution, ICG tends to form polymers if the solution concentration is high and monomers if the concentration is low. For this reason the dye is dissolved in water for intravenous injections.
Peak absorption of the monomers and polymers is observed at 785 and 690 nm, respectively. Binding to proteins in turn elevates the absorption spectrum to 810 nm, since polymer formation decreases. The emission spectrum in aqueous solution is 810-820 nm, though in blood it shifts 10 nm towards longer wavelengths. The ICG molecule can be considered active at a wavelength of between 790 and 830 nm.
Following intravenous administration, ICG binds to blood lipoproteins (fundamentally phospholipids) in a proportion that can reach 98%(11-13). The emitted fluorescence intensity increases as a result of such binding. Elimination takes place in the liver, with secretion in bile.
The adverse reactions are similar to those observed with fluorescein, but are less frequent(10). In 1994, explorations with ICG documented a 0.15%, 0.2% and 0.05% incidence of mild, moderate and severe reactions, respectively(10). The dye should not be used in patients with shellfish or iodine allergies, liver disease, and end-stage renal disease. No studies in animal models have been made regarding use of the dye in pregnancy. However, current practice patterns regarding the use of ICGA in pregnant woman may be unnecessarily restrictive(14).
Exploration is carried out in the same way as in FA, though with the corresponding excitatory light and barrier filter. The contrast is injected and images are obtained documenting the fluorescence passing through the ocular fundus during the filling phases, and posteriorly after 1, 3, 5, 10, 20 and 30 minutes.
The ICG molecule has biophysical properties that make it useful for visualizing the choroidal circulation. The dye is excited by light close to infrared, increasing its penetration through the retinal pigment epithelium (RPE), melanin, xanthophyll pigment and thin blood layers. ICG circulates almost entirely bound to plasma proteins, and gradually diffuses across the choriocapillaris – thus allowing better visualization of its circulation within the choroidal vessels. These properties make it possible to visualize CNV beneath blood layers, exudates or RPE detachments (PEDs)(15-16) by differentiating (in the latter case) between the hypofluorescent serous component and the hyperfluorescent vascular component (Figure 1).
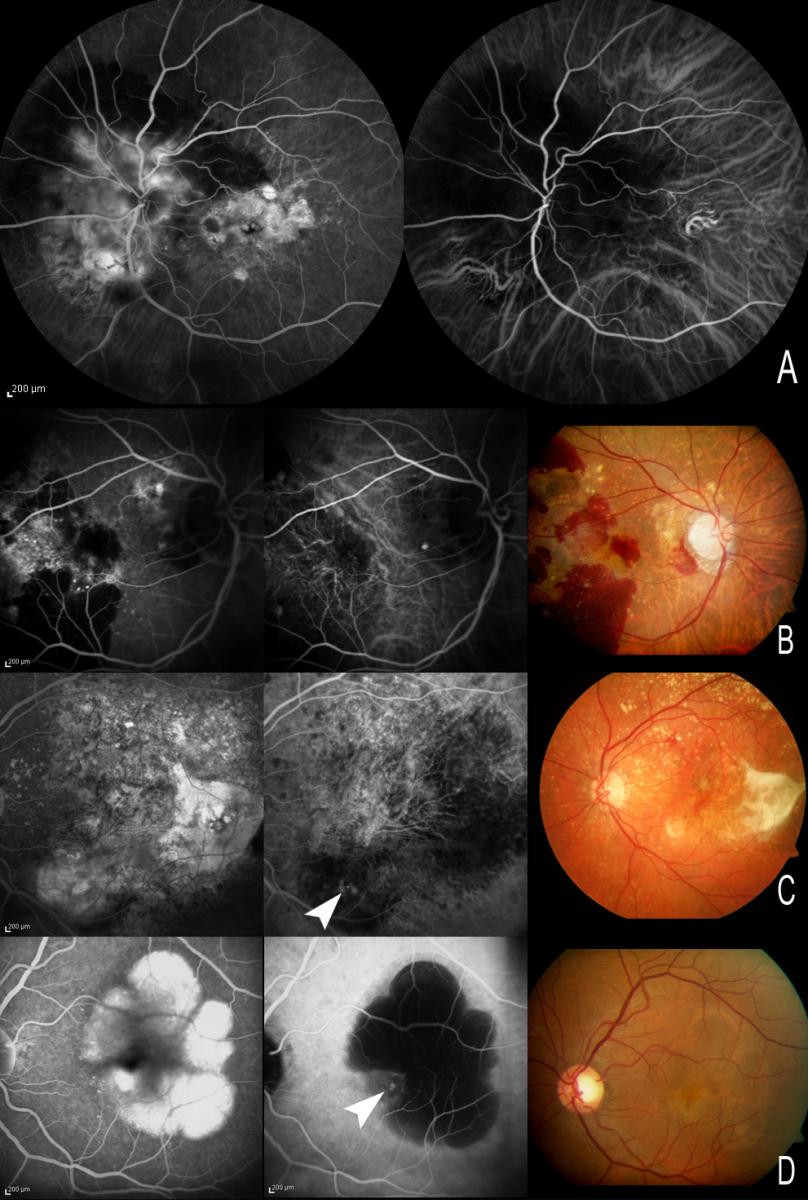
Figure 1. (A) The ICG molecule has biophysical properties that make it useful for visualizing the choroidal circulation. (B, C and D) These properties make it possible to visualize choroidal neovessels beneath blood layers, exudates or RPE detachments (PEDs) by differentiating (in the latter case) between the hypofluorescent serous component and the hyperfluorescent vascular component. With FA, both the serous and the vascular component appear hyperfluorescent, and the limits of CNV may be masked as a result. A and B. Visualization of the choroidal circulation under thin blood layers. (C) Hot spots visible in an area of exudation. (D) PED with small CNV at the margin of the lesion. With FA, both the serous and the vascular component appear hyperfluorescent, while with ICGA only the vascular component of PED appears hyperfluorescent.
The most important contribution of ICGA to the study of CNV in AMD is its capacity to detect choroidal new vessels which in some cases remain occult when using FA(15,16). Following the development of ICGA in the early 1970s(17,18), two decades went by before scanning laser ophthalmoscopy (SLO) and digital angiography made it possible to improve ICGA visualization(19-21) and registry, and routine application of the technique in clinical practice.
In the 1990s, different studies(22) showed that ICGA allows the visualization of OCNV, the most frequent type of CNV in the context of neovascular AMD. The capacity of ICGA to locate new vessels particularly in those cases with associated PEDs made it possible to increase the number of cases of CNV amenable to laser photocoagulation therapy – the latter being the only treatment option available at that time for such patients.
Until the end 1990s, the visualization and delimitation of neovascular membranes was regarded as very important, due to the inclusion of extra- and juxta-foveal locations as an indication for laser photocoagulation(22). In this context, the use of ICGA was of great help due to its capacity to delimit OCNV. In 1992, Yannuzzi et al.(23) showed that late frames of ICGA allowed visualization of the entire choroidal neovascular membrane(22). Before this time, ICGA had already been found to be useful in detecting CNV in cases of block caused by media opacity(24,25).
ICGA served to guide photocoagulation of OCNV without classic CNV, particularly when this OCNV was located inside or at the margin of PED(26,27). The images obtained with this technique in such cases of vascularized PEDs allowed the individualization of a new form of CNV known as chorioretinal anastomosis, later termed retinal angiomatous proliferation (RAP) or type 3 neovascularization(28-30). Scanning laser ophthalmoscopy (SLO), more than vid
In his description, Yannuzzi (58) defended this retinal onset of neovascularization, while Gass (38) suggested that the process starts in the choroid as occult CNV.
When FA is used, RAP manifests as an occult form of CNV with PED and perilesional microhemorrhage and frequent intraretinal edema, associated to neurosensory retinal detachment (NSRD) and RCA in advanced stages.
eoangiography, truly improved the quality of the early frames of ICGA, making it possible to record a dynamic sequence of choroidal filling and improving visualization of this particular neovascular network corresponding to chorioretinal anastomosis(22).
Using SLO, other authors also described the use of high-speed ICGA for visualizing the feeding vessels in cases of OCNV, allowing their selective photocoagulation without the need to cover the entire membrane(31-33). However, the usefulness of ICGA in these cases is limited, since in many cases there is not only one but several feeding vessels; there may be failures in identification; and laser photocoagulation is often not enough to ensure definitive sealing of the afferent vessel(34).
The introduction of verteporfin photodynamic therapy (V-PDT) in the late 1990s allowed the treatment of subfoveal CNV. Different randomized clinical trials were performed, such as the TAP (Treatment of Age-Related Macular Degeneration with Photodynamic Therapy) and VIP (Verteporfin in Photodynamic Therapy) trials, which respectively, included patients with classic and occult CNV(35,36). New vessels were classified according to the FA findings, while ICGA was not performed because PDT became standard care treatment for classic subfoveal CNV.
Anti-VEGF therapy inaugurated a new era in the management of exudative AMD, because it was the first treatment to improve the mean visual acuity of eyes treated with intravitreous injections of anti-VEGF(37,38), which proved effective in both classic and occult subsets of CNV. In randomized clinical trials, CNV was classified according to the FA findings, and ICGA did not form part of the protocol. However, eyes with PEDs were not included in the initial randomized clinical trials, and it is known that such conditions show a poorer treatment response and predispose to RPE rupture(39).
New developments of OCT include better visualization of the choroid(40-43), and this noninvasive technology reduced the use of angiographic testing in routine clinical practice in patients with exudative AMD in which fixed-regimen monthly intravitreous reinjection are used. OCT brings another classification of the neovascular type secondary to AMD: type I is a lesion which grows under the RPE, type II is subretinal and type III corresponds to RAP.
ICGA remains necessary when the diagnosis proves uncertain despite FA and OCT; in certain cases of OCNV with PEDs (particularly in cases of suspected IPCV and RAP) or conditions such as central serous chorioretinopathy (CSC), which may require a different therapeutic approach from that used in neovascular AMD; and in the re-evaluation of non-responding patients.
Due to the economical and medical care burden implied by this type of treatment, an early and correct diagnosis is very important in order to prescribe adequate patient management.
Current Importance of ICGA in AMD
Nonexudative (Dry) AMD
In nonexudative AMD, ICGA has been used to improve drusen classification(44,45); to facilitate diagnosis of basal laminar drusen and the associated subretinal deposits(46); and to improve understanding of reticular pseudodrusen(47,48), due to the affinity of some lesions for this dye.
However, ICGA is not routinely used to study dry AMD(22), except in cases of drusenoid PED to confirm the absence of CNV (Figure 2).
Drusenoid PED could be defined as the progressive increase and confluence of small soft drusen. These appear as yellow-gray spots, sometimes with a characteristic hyperpigmented ring, due to their slow and progressive growth. OCT in application to these drusenoid forms allows us to identify elevations of the pigment epithelium, sometimes variable in number and sometimes as a single and larger PED, filled with hyper-reflecting material.
Drusenoid PEDs require no treatment, since they do not imply the presence of neovascular activity, and ICGA is important for establishing this differentiation (Figure 2).
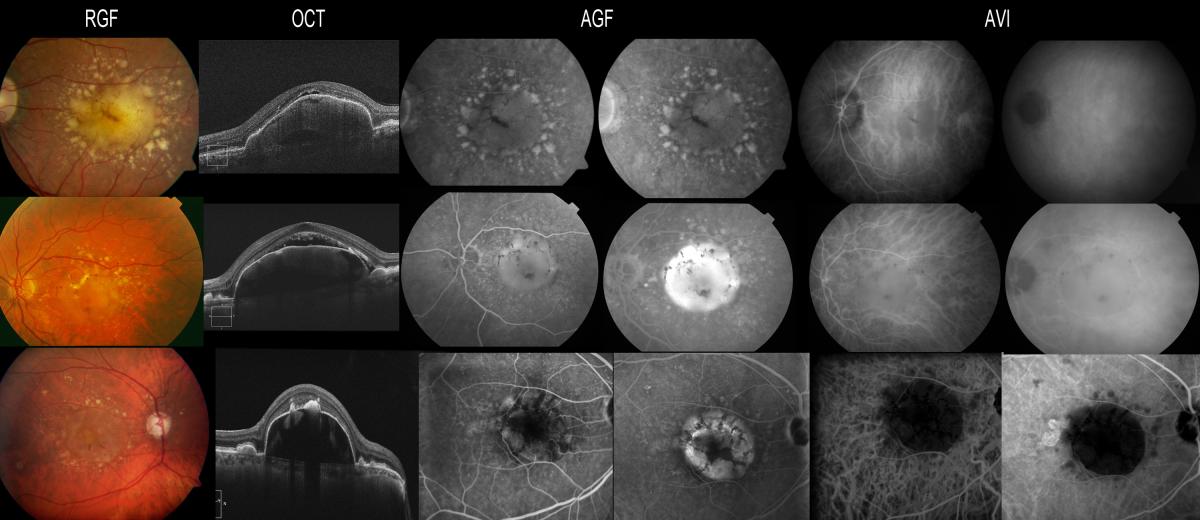
Figure 2. Drusenoid PEDs require no treatment, since they do not imply the presence of neovascular activity, and ICGA is important for establishing this differentiation. Drusenoid PEDs appear hyperfluorescent in the fluorescein study, while ICGA allows the exclusion of CNV if there is no hyperfluorescent component associated to the hypo- or isofluorescence of the PED.
Recently, ICGA has been reported to be useful for evaluating the atrophic areas present in Stargardt disease, in comparison with the atrophy of dry AMD(49). The hypocyanescence evidenced by ICGA in the atrophic areas (“dark atrophy”) has been shown to be more frequent in Stargardt disease than in atrophic AMD – suggesting a possible selective damage of the choriocapillaris in Stargardt disease.
Exudative (Wet) AMD
Using ICGA, CNV has been reclassified into three different morphologic types: focal spot or “hot spot”, plaques (well or poorly defined), and mixed (i.e., a combination of the previous two)(50,51). These alterations may be located at the margin of the lesion (marginal spot), above the lesion (overlying spot), or at a distance from the lesion (remote spot) (Figures 3-4).
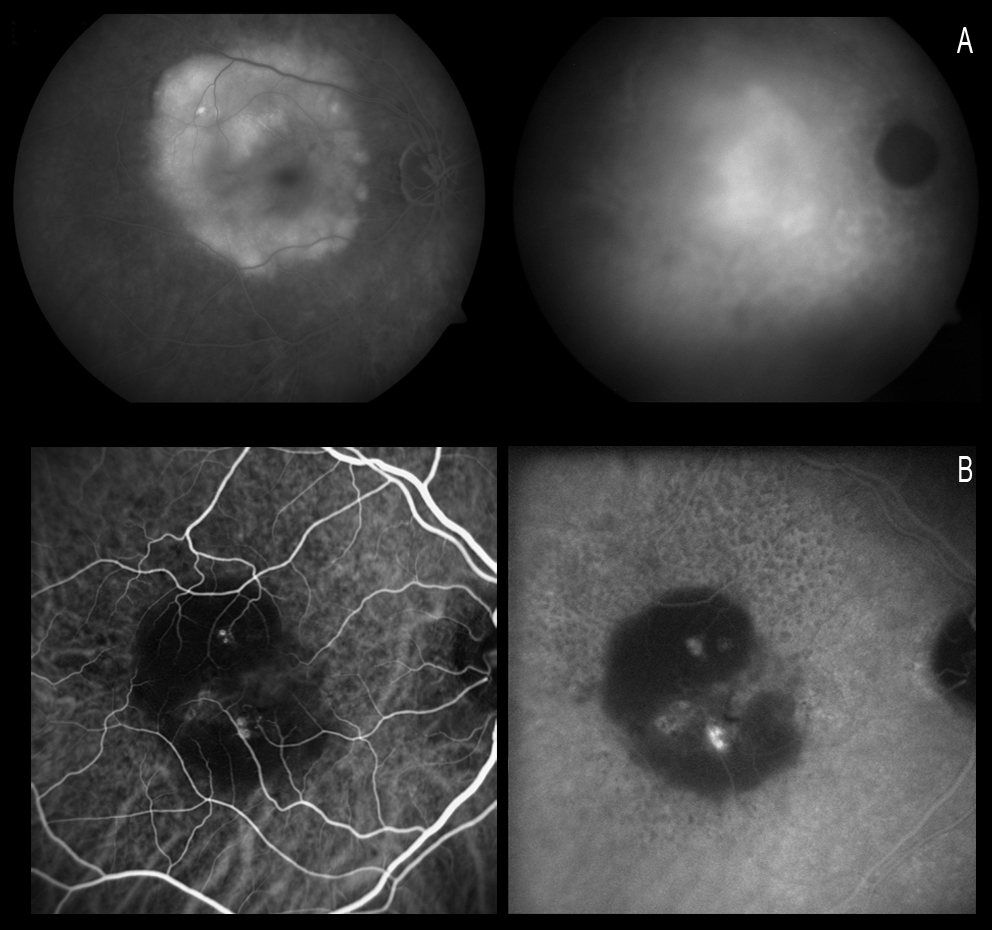
Figure 3. Using ICGA, CNV has been reclassified as focal spots (or hot spots), plaques or mixed forms (a combination of the first two forms). Figure A shows a plaque with poorly defined margins, in the late phases of ICGA. Figure B shows two hot spots (the lower spot being more active).
Yannuzzi(23,60) reported that hypofluorescence is maintained throughout the duration of the test in non-vascularized PEDs. On analysing these changes in serous PED with CNV, this author established two well defined groups in 96% of the patients: (a) cases where ICGA shows a solitary and well defined hyperfluorescence spot which he refers to as focal CNV or a “hot spot”; and (b) a “plaque CNV”, which is a larger and poorly defined lesion with less intense fluorescence. In the remaining 4% of the cases the author observed no CNV with ICGA.
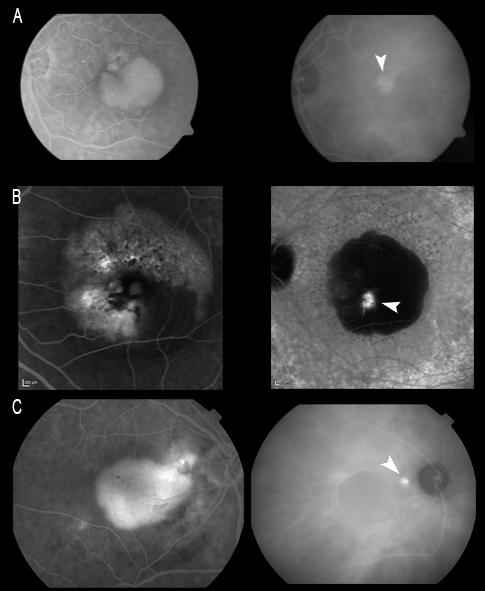
Figure 4. CNV may be located: (A) At the margin of the lesion (marginal spot); (B) Above the lesion (overlying spot); or (C) At a distance from the lesion (remote spot).
Plaques were found to be the most common type (61% of the cases) and had a poor visual prognosis, whereas the focal spots or “hot spots” (29%) had a better prognosis, and they were considered to be potentially treatable by ICG-guided laser photocoagulation(50,52).
Moreover, ICGA is useful to identify underlying causes of spontaneous submacular hemorrage, allowing for different approaches that may improve outcomes and safety(53).
Classic CNV
Classic CNV is better delimited by FA than by ICGA(54,55). ICGA does not offer relevant supplementary information unless associated OCNV is present. As a result, it is likewise not routinely used to study classic CNV.
Occult CNV (OCNV)
The term “occult” refers to a type of CNV that is difficult to visualize, analyse and localize on FA. This difficulty is due to their polymorphic features, but especially to the fact that they are ill-defined and ill-delimited(56). The fundamental advantage of ICGA is that it allows early detection and localization of OCNV and analysis of the filling and staining pattern of the CNV network of the lesions.
OCNV is very common, and most cases of CNV in AMD (60-85%) correspond to this type(57,58).
These cases may present a variable course:
- A slow and almost asymptomatic course for many months.
- A course characterized by exacerbation episodes.
- A slowly progressing course with impairment of visual acuity, metamorphopsia and gradual growth of the lesion.
The natural outcome of the disease is generally poor, with severe central vision loss due to hemorrhages, sometimes associated with serous PEDs and/or tearing of the pigment epithelium (RPE Tear), and eventually, fibrous proliferation with formation of a fibrous scar.
OCNV can be grouped into two main types(56):
- OCNV without serous PED;
- OCNV associated with serous PED.
The classification proposed by the MPS (Macular Photocoagulation Study) investigators is similar and based on FA:
- Type I: Fibro-vascular PED (Fibro-vascular pigment epithelium detachment with irregular and poorly demarcated hyperfluorescence);
- Type II: Late leakage of an undetermined source (without PED and without classic CNV).
RAP or type 3 neovascularization in turn is added to the above(29,30).
The importance of ICGA in the study of OCNV is that it can afford an early diagnosis of the disease in cases that prove doubtful with FA and OCT particularly cases involving OCT based differential diagnosis with neurosensory detachment, which can also occur in CSC.
1. OCNV without serous PED
FA shows irregular and poorly delimited hyperfluorescence with late phase diffusion suggestive of OCNV. OCT in these cases is essential for evaluating the degree of activity due to the presence of neurosensory detachment and thickening of the pigment epithelium membrane – choriocapillary band, which is more suggestive of OCNV than of CSC in the absence of leakage points in FA. OCT is also fundamental for the follow-up of these patients.
2. OCNV with serous PED
Serous or serohematic PED appears in the ocular fundus as a yellow-orange elevation with margins that are very well defined by the solid adherence of the pigment epithelium to Bruch’s membrane at this level.
Serous PED may be avascular or vascular. For all eyes with PEDs, the purpose of the initial evaluation is to identify or exclude the presence of CNV, as this feature will change the treatment in the anti-VEGF era. ICGA is very useful in these cases for establishing the presence or absence of associated neovascularization, since such CNV may be masked by the hyperfluorescence of PED when FA is used (Figure 5).
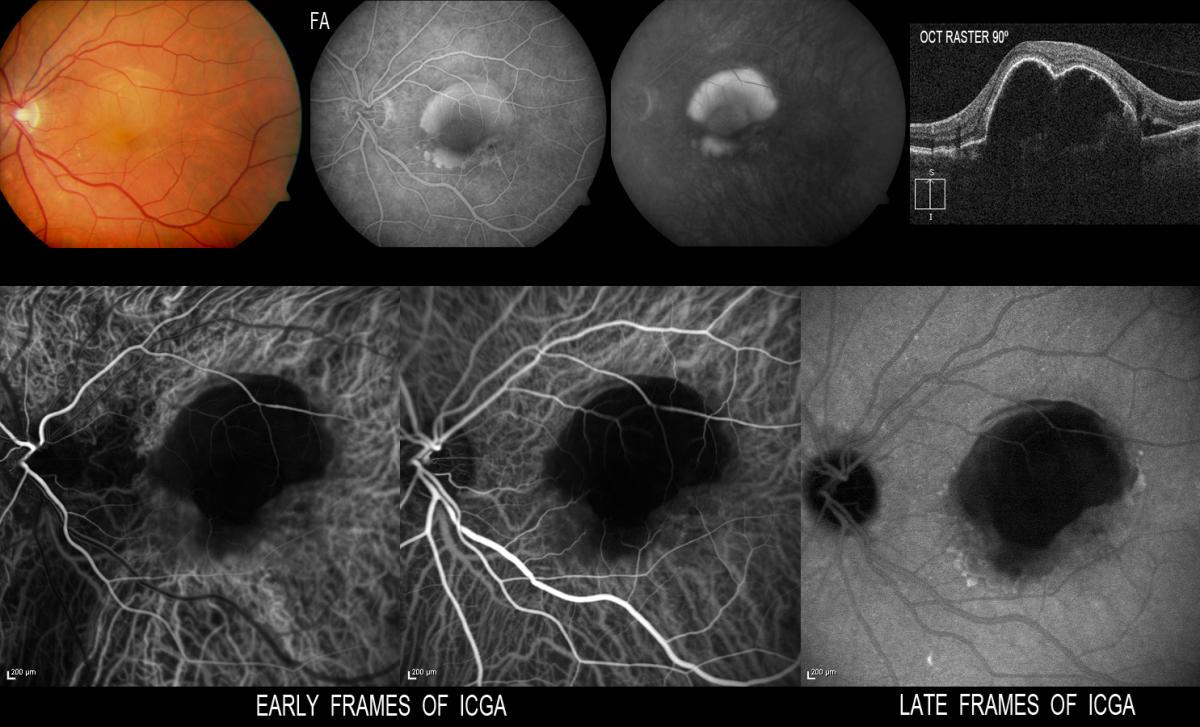
Figure 5. ICGA is very useful in these cases for establishing the presence or absence of associated neovascularization, since CNV may be masked by the hyperfluorescence of PED when FA is used. When FA proves inconclusive, ICGA may confirm the absence of neovascularization.
When ICGA is used, PED appears as a hypofluorescent lesion in all phases of the angiogram. In some cases, as a result of the accumulation of lipids on Bruch’s membrane, the lesion may have an isofluorescent or even somewhat hyperfluorescent appearance, while in contrast CNV always appear hyperfluorescent. Gass(59) described a blood or pigment meniscus in a zone of the PED, forming a notch that affords a kidney-like shape and which represents the zone of firm adherence between the CNV and the pigment epithelium. This notch which gives the PED a kidney shape allows us to suspect where the CNV is located.
Yannuzzi(23,60) reported that hypofluorescence is maintained throughout the duration of the test in non-vascularized PEDs. On analysing these changes in serous PED with CNV, this author established two well defined groups in 96% of the patients: (a) cases where ICGA shows a solitary and well defined hyperfluorescence spot which he refers to as focal CNV or a “hot spot”; and (b) a “plaque CNV”, which is a larger and poorly defined lesion with less intense fluorescence. In the remaining 4% of the cases the author observed no CNV with ICGA.
ICGA is a very useful diagnostic technique and superior to FA in diagnosing and classifying CNV associated to serous PEDs. As can be seen in the figures, all types of neovascularization can be found associated to serous PED: classic, occult, RAP, and also quite often associated to IPCV (as shown in the Figure 6).
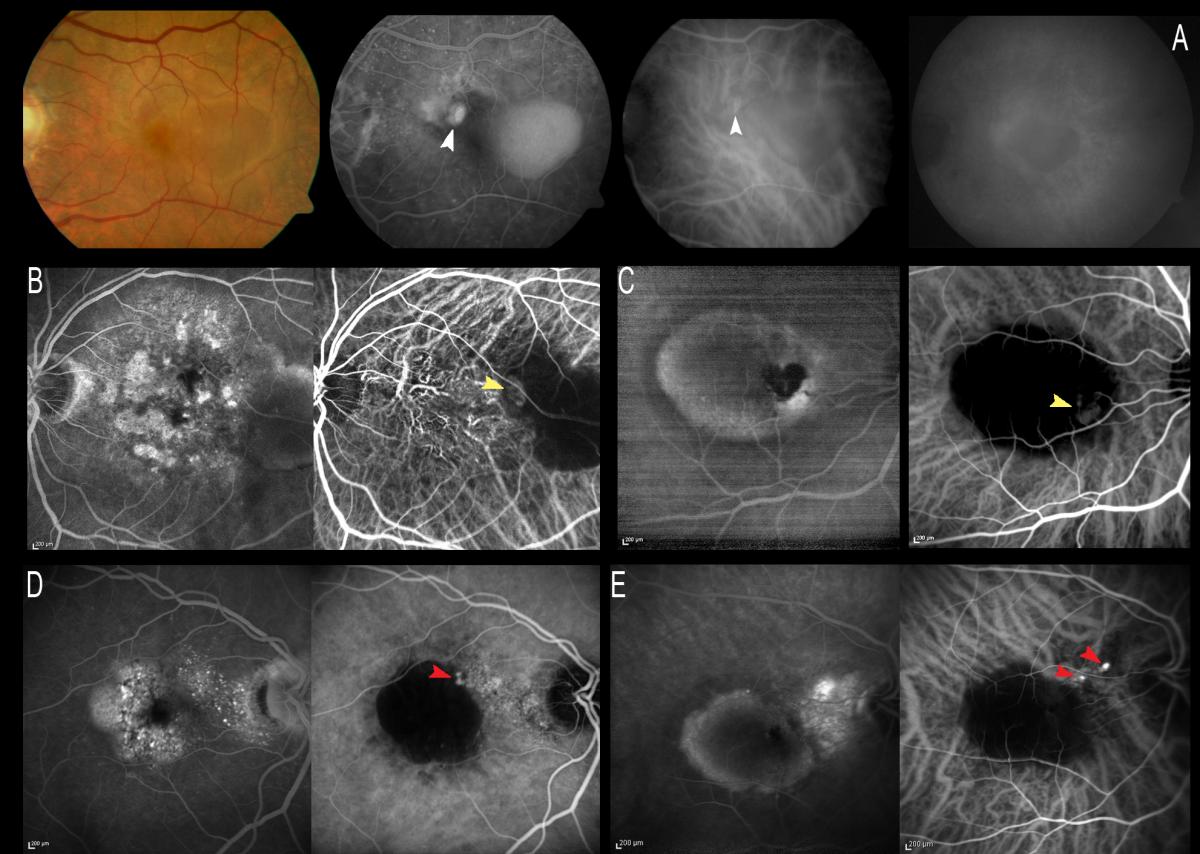
Figure 6. ICGA is very useful for diagnosing and classifying CNV associated to serous PED, due to its capacity to distinguish between the serous and the vascular component. All types of associated CNV may be found. Figure A shows classic CNV which is better visualized with FA in the early phases, since it is masked in the late phases by the hyperfluorescence of the entire lesion. The rest of the cases involve OCNV, where ICGA can show hot spots on the black image of the PED associated to RAP (yellow arrows) or polyps (red arrows).
OCTA used to enhance the detection of neovascularization could be possible in some cases where neovascularization is not clearly detected with OCT or dye-based angiographies. Nowadays, it could be interesting to use a multimodal approach in these cases(61).
Retinal Angiomatous Proliferation (RAP)
RAP has been described as a variant of exudative AMD characterized by the presumed retinal origin of CNV(62) (Figure 7).
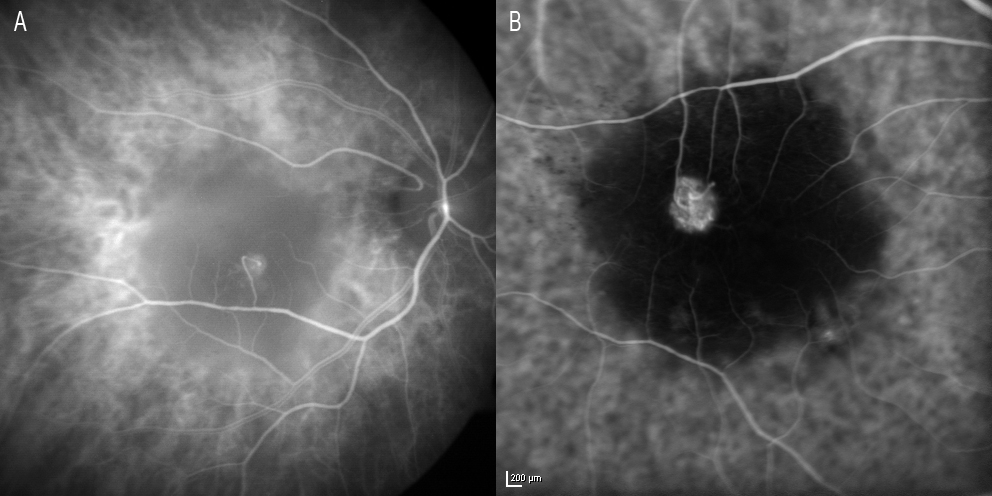
Figure 7. RAP has been described as a variant of exudative AMD characterized by the presumed retinal origin of CNV. (A) Image of retinal communication under Zeiss FF 450 plus IR (Carl Zeiss Meditec, Jena, Germany). (B) HRA (Heidelberg Retinal Angiography 2) image (HRA2, Heidelberg Engineering, Germany).
The pathogenesis and classification of RAP remain subject to debate(63).
Yannuzzi (62) was the first to describe this condition, establishing three different stages on the basis of the clinical and angiographic findings:
- STAGE I. Proliferation of intraretinal capillaries; intraretinal neovascularization;
- STAGE II. Subretinal new vessels without PED (IIA) or with PED (IIB);
- STAGE III. Choroidal neovascularization (CNV);
- Such CNV often consolidates as retinochoroidal anastomosis (RCA), which posteriorly has been classified as:
- STAGE IV. Retinochoroidal anastomosis (RCA) (64,65).
RAP represents approximately 12-20% of all cases of exudative AMD (30,66,67), and currently the term type III neovascularization has been proposed for this condition, precisely in order to distinguish it from other types of CNV in exudative AMD (28,65,68).
This disorder is known to show a different natural course and response to treatment compared with other forms of AMD(69,70,71). The existence of retino-retinal anastomosis (RRA) and RCA(62,64) is characteristic of RAP type lesions and contributes to the presence of high-flow vascular alterations with double retinal and choroidal circulation. This and the presence of associated PED and cystoid macular oedema in the more advanced stages complicates the treatment of this disease(72) (Figure 8).
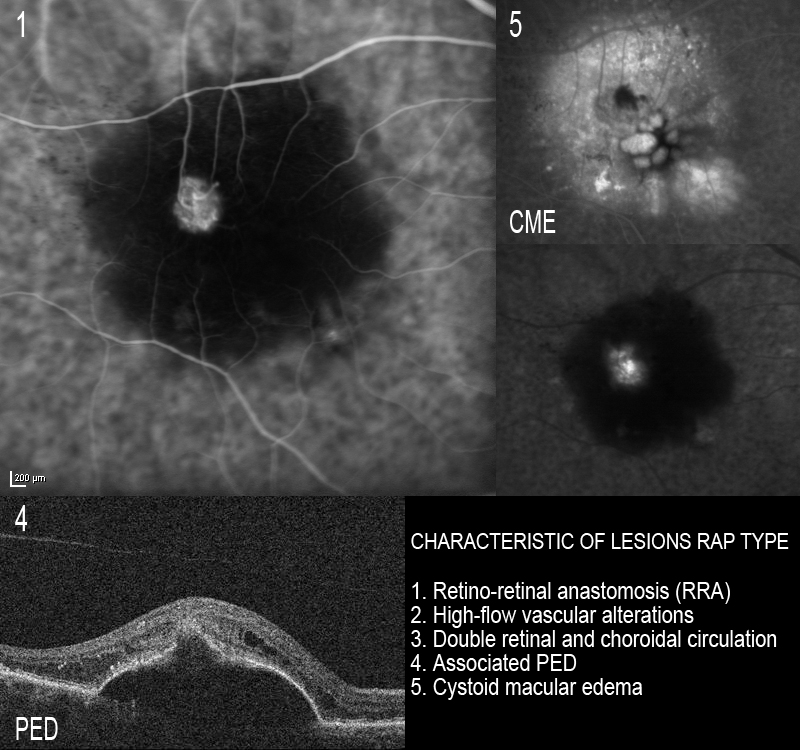
Figure 8. The existence of RRA is characteristic of RAP type lesions and contributes to the presence of high-flow vascular alterations with double retinal and choroidal circulation. This, and the presence of associated PED and cystoid macular edema, complicates the treatment of this disease.
In some cases, ICGA in the early phases allows us to identify RRA with a nutrient vessel and a draining vessel(73) – this representing a retinal neoangiogenic process that subsequently spreads to the subretinal space (Figure 8).
In some cases, ICGA in the early phases allows us to identify retino-retinal anastomosis with a nutrient vessel and a draining vessel (69) – this representing a retinal neoangiogenic process that subsequently spreads to the subretinal space (Fig.8).
In his description, Yannuzzi(62) defended this retinal onset of neovascularization, while Gass(40) suggested that the process starts in the choroid as OCNV.
When FA is used, RAP manifests as an occult form of CNV with PED and perilesional microhemorrhage and frequent intraretinal edema, associated to neurosensory detachment and RCA in advanced stages.
ICGA allows us to study the existence of single or multiple hot spots at neovascularization level. This is very useful in the earliest stages, especially to the effects of treatment, if V-PDT is to be associated. ICGA also allows us to observe spot persistence or disappearance after treatment, as well as the reappearance of spots in the case of patients who are re-evaluated due to a lack of response or resistance to therapy. In some early stage cases, ICGA can reveal the characteristic retino-retinal vascular communication in the early phases of the angiogram (Figure 9).
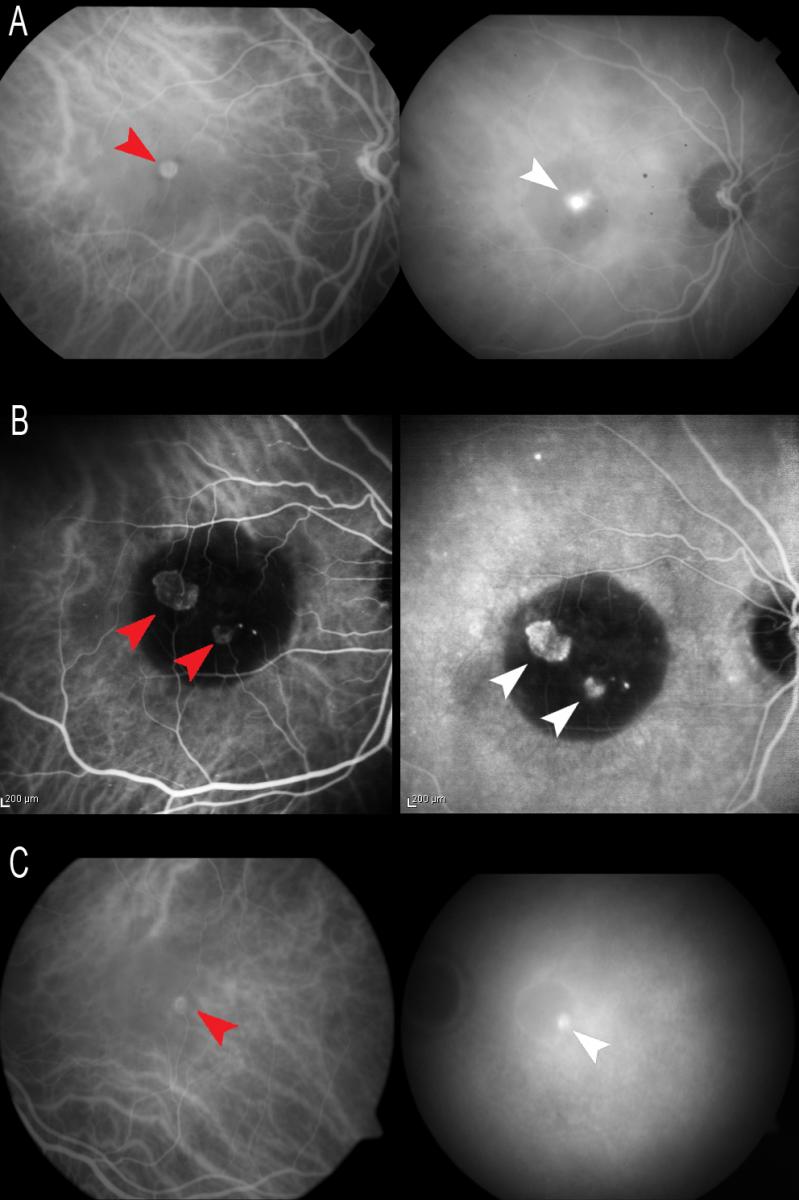
Figure 9. ICGA allows us to study the existence of single (A and C) or multiple (B) hot spots at neovascularization level (white arrows). In some early stage cases, ICGA can reveal the characteristic retino-retinal vascular communication in the early phases of the angiogram (red arrows).
It is important to distinguish RAP from other forms of AMD, not only in view of the poor vision prognosis if exhaustive patient follow-up and correct treatment are not carried out, but also due to the high risk of neovascularization in the other eye(70,74,75). In this context, ICGA helps us to establish a correct diagnosis (Figure 10).
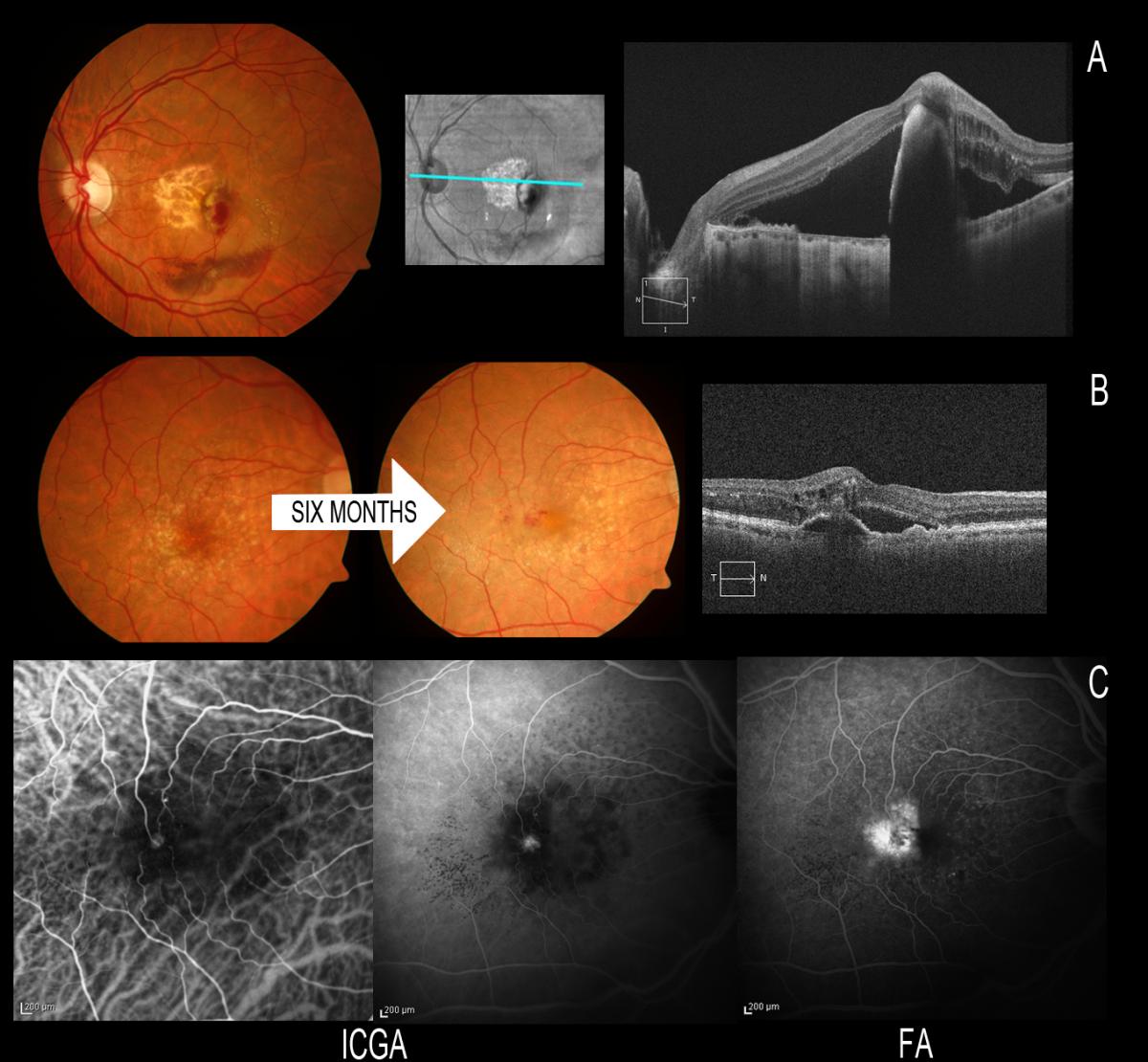
Figure 10. It is important to distinguish RAP from other forms of AMD due to the high risk of neovascularization in the other eye when the disease is present in one eye. (A) RAP first manifesting in the left eye with spontaneous rupture of the RPE; we must keep a very close follow-up because of a possible involvement of the fellow eye. (B) After 6 months the fellow eye is affected, and ICGA helps us to establish a correct early diagnosis. We can see the communication in early phases and the small hot spot in late phases of ICGA.
The precise mechanism giving rise to this peculiar form of vascularization remains unclear, though some authors recently have suggested that there is ischemia at external retinal level, induced by diminished choroidal perfusion(76-78).
RAP requires more intensive management, often involving combined treatments, in order to control the activity of the disease(70, 79-82). Recently, favourable results have been published with the use of anti-VEGF drugs, though with a high recurrence rate after suspending the treatment(63,83-97).
V-PDT has been associated to anti-VEGF drugs and to intravitreous triamcinolone (IVT), with promising results(98-114). Combined treatment proves effective in maintaining or improving patient vision and in reducing exudation, without long-term adverse effects(110). Rouvas et al.(112) have reported stabilization in patients administered with ranibizumab alone and in patients administered with ranibizumab associated to V-PDT, with functional and anatomical improvements in the patients subjected to V-PDT with IVT.
Regarding the combined treatments, it must be taken into account that there is an increased risk of developing atrophy in patients with RAP subjected to V-PDT(112). Frequent geographic atrophy has been described in these patients after the treatment(77), and recently there have also been reports(78) of specific changes in the choroid and in drusen distribution, which may have long term diagnostic and therapeutic implications (Figure 11).
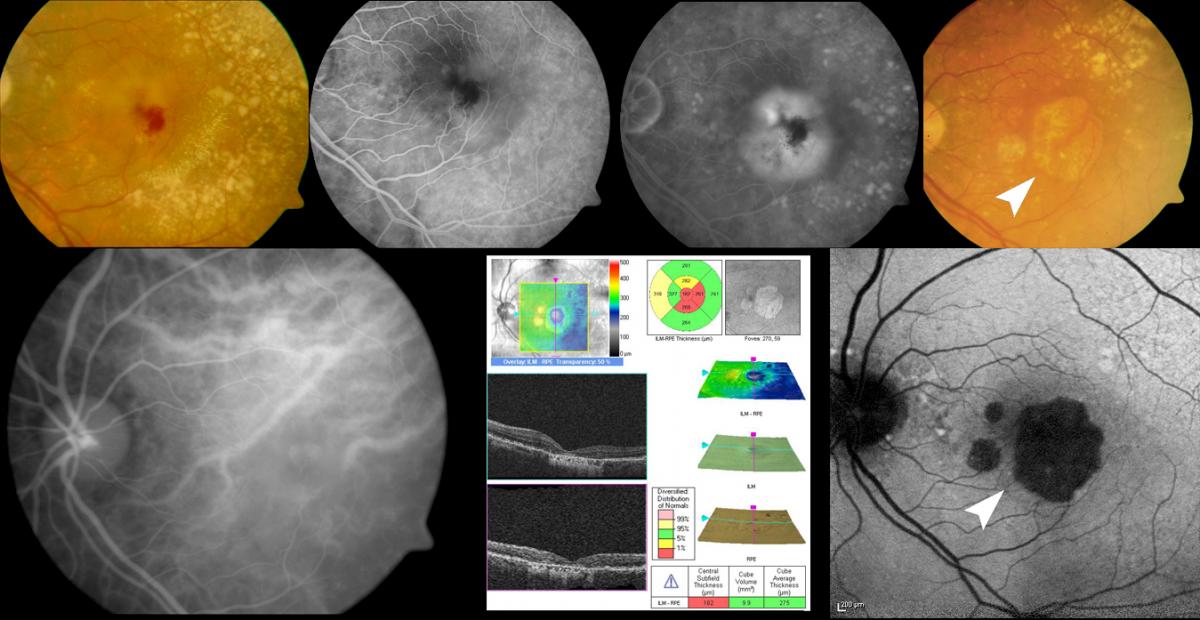
Figure 11. Regarding the combined treatments, it must be taken into account that there is an increased risk of developing atrophy in patients with RAP subjected to V-PDT. Frequent geographic atrophy has been described in these patients after the treatment (white arrow).
In coincidence with other authors(111), we perform ICGA-guided PDT in some patients in stages I and II, with the smallest possible spot covering the neovascularization, associated to anti-VEGF drugs. This technique would use smaller spots, thereby contributing to lessen the risk of long-term atrophy in these patients (Figure 12).
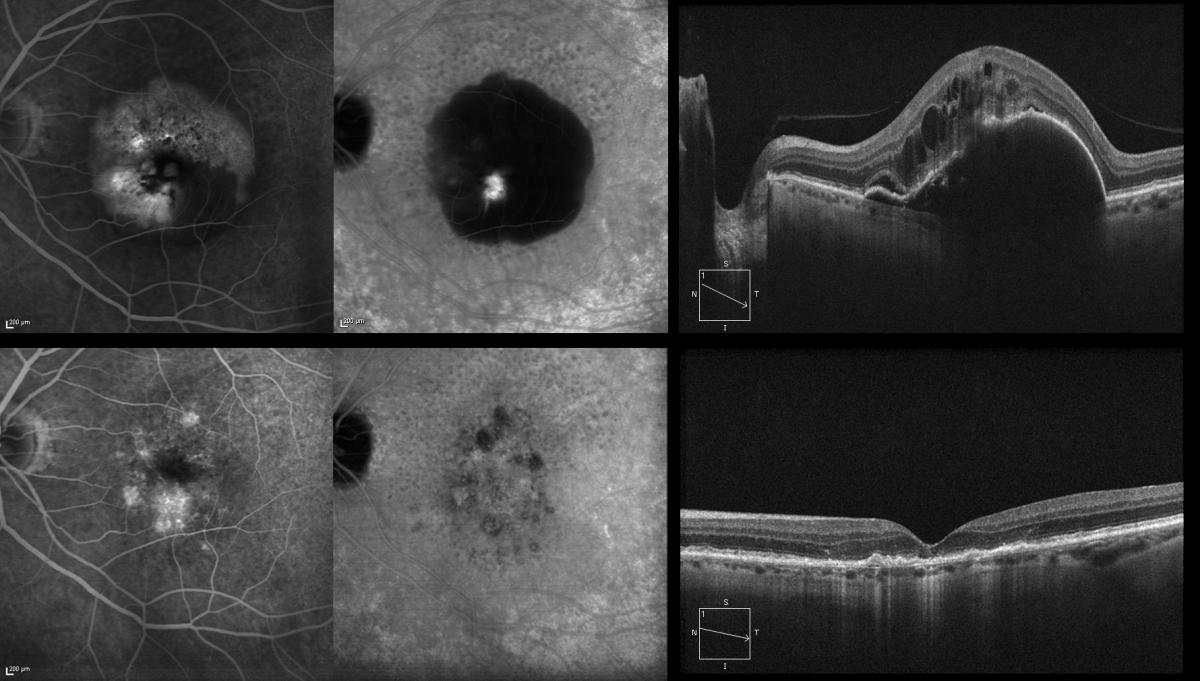
Figure 12. We perform ICGA-guided PDT in some patients in stages I and II, with the smallest possible spot covering the neovascularization, associated to anti-VEGF drugs. This technique would use smaller spots, thereby contributing to lessen the risk of long-term atrophy in these patients. The figure shows the outcome, three years after the treatment.
It is also known that the prognosis is poorer in cases of large PEDs, where pigment epithelial rupture may be more frequent after treatment(115-117) (Figure 13), and that better functional results are obtained when these patients are diagnosed and treated in early stages of the process(118). The diagnosis should be, therefore, established as soon as possible, and ICGA is very important to this effect (Figure 14).
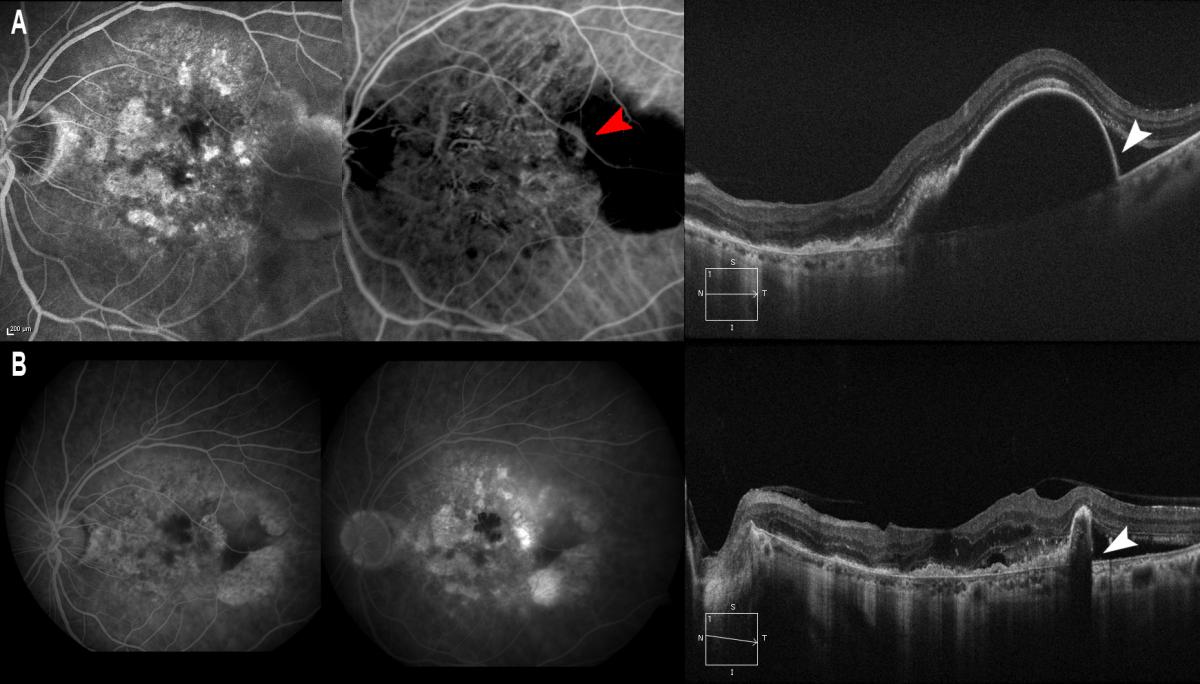
Figure 13. It is also known that the prognosis is poorer in cases of RAP (A, red arrow) with large PEDs, where pigment epithelial rupture may be more frequent after treatment. (A) PED is observed/marked with a thin wall (A, white arrow) where pigment epithelial rupture occurs after intravitreous injection of ranibizumab (B, white arrow).
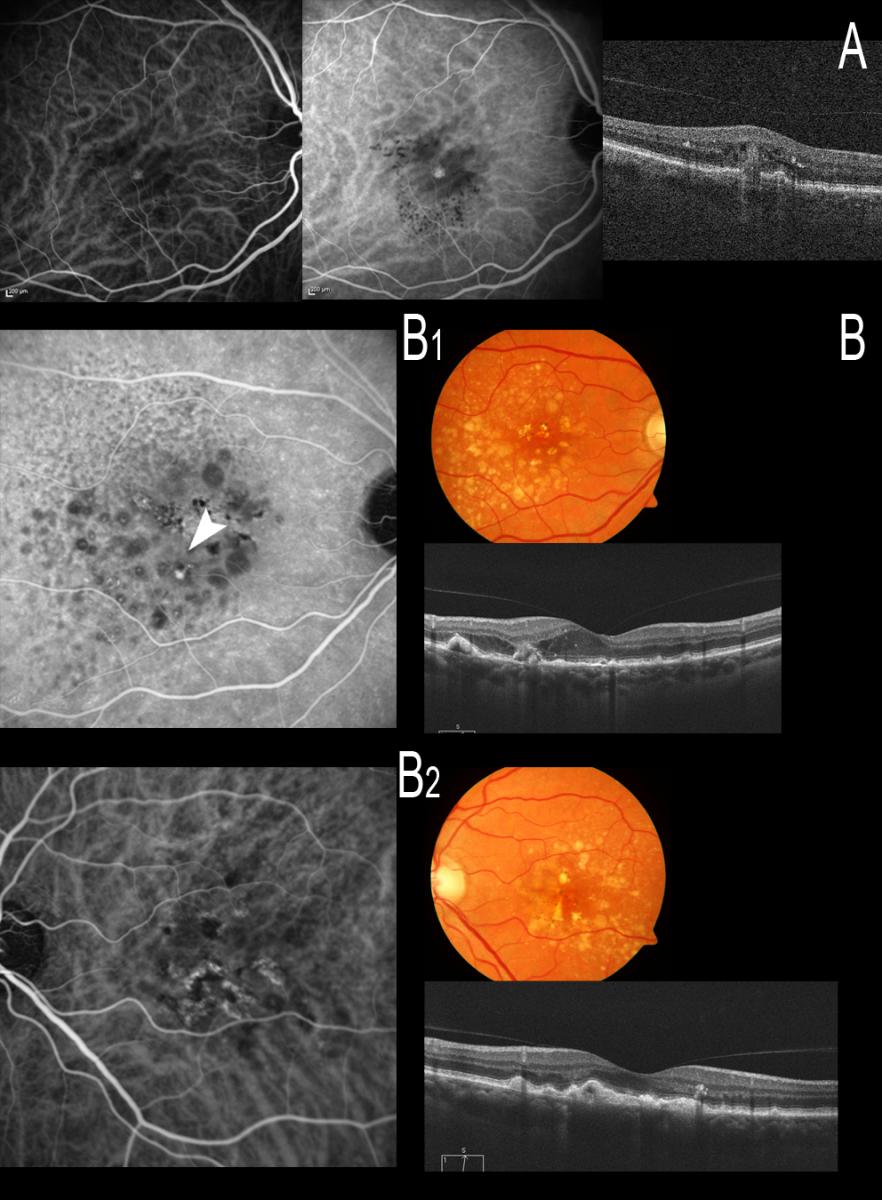
Figure 14. The diagnosis therefore should be established as soon as possible, and ICGA is very important to this effect. (A) Stage I RAP is observed, easily identifiable with ICGA. (B) A patient with bilateral drusen where ICGA identifies an incipient communication (B1, white arrow) that produces alterations with intraretinal edema as evidenced by OCT (B1); ICGA proves negative in the other eye, however (B2).
OCT may be used to diagnose type 3 neovascularization in patients with neovascular AMD with relatively high concordance compared with ICGA-based diagnosis(119).
Idiopathic Polypoidal Choroidal Vasculopathy (IPCV)
IPCV is characterized by the presence of a network of abnormal choroidal vessels with aneurysmal dilatations in the form of polyps visualized as red-orange nodules in the ocular fundus and located mainly at peripapillary level and in the macular zone(120-123). These choroidal vascular lesions can produce serous exudation and hemorrhage that have been associated to recurrent serohematic PEDs and neurosensory detachment(122,124-128) (Figure 15).
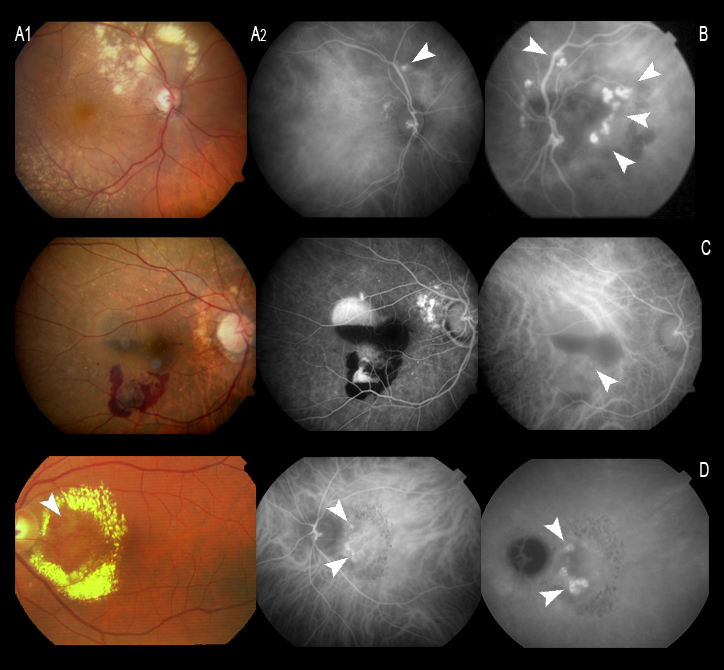
Figure 15. (A and B) Peripapillary polyps with a large exudative component at superior temporal arcade level in the first patient. (C) Serohemorrhagic PED at macular level, with a small polyp, evidenced by ICGA (C, white arrow). (D) Circinate macular lesion centered by a network of polyps (D, white arrows).
The vascular lesions of IPCV can simulate an occult or minimally classic membrane with FA, while ICGA clearly reveals the polypoidal lesions and anomalous vascular network within the choroid. In concordance with this, a literature review published by Stanga et al. concluded that ICGA is highly recommended for the identification of choroidal lesions in patients with IPCV(51) (Figure 16).
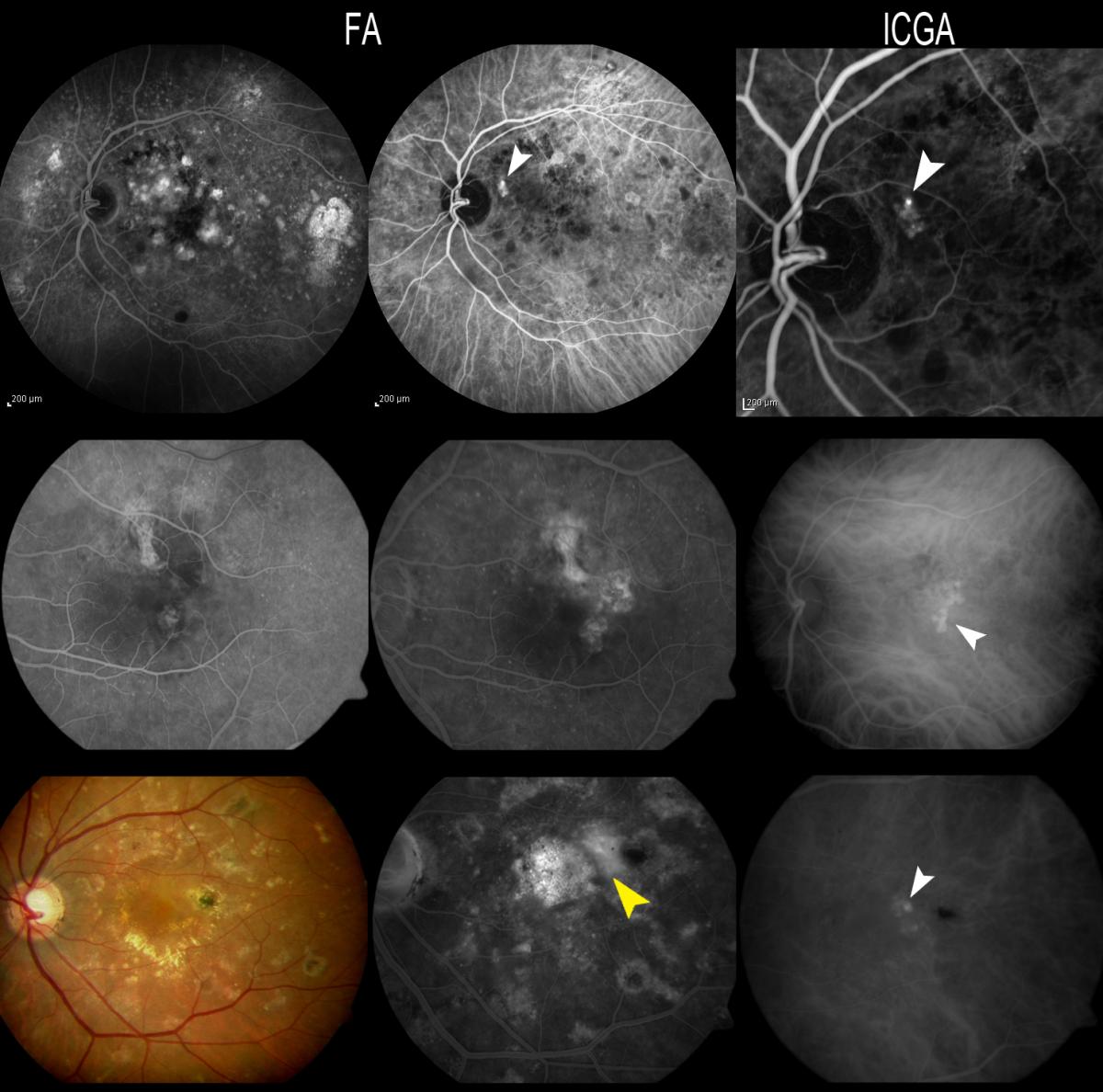
Figure 16. Patients with OCNV in FA, where the polyps are seen by ICGA. The three patients responded poorly to anti-VEGF therapy and where therefore re-evaluated due to suspected IPCV. In case C, FA reveals a hyperfluorescent vascular PED (yellow arrow), while only the polyps at the margin of the PED appear hyperfluorescent with ICGA (white arrow).
ICGA is able to identify two elements in these anomalies: polypoidal structures projecting from the internal choroid towards the retina, and a branched choroidal vascular network (BVN) manifesting with early vascular hyperfluorescence(129-131). The underlying pathogenesis remains subject to debate (Figure 17). In this sense, some authors suggest that the polyps are a form of CNV(132-135), while others regard them as an alteration of the internal choroidal vessels(136-138).
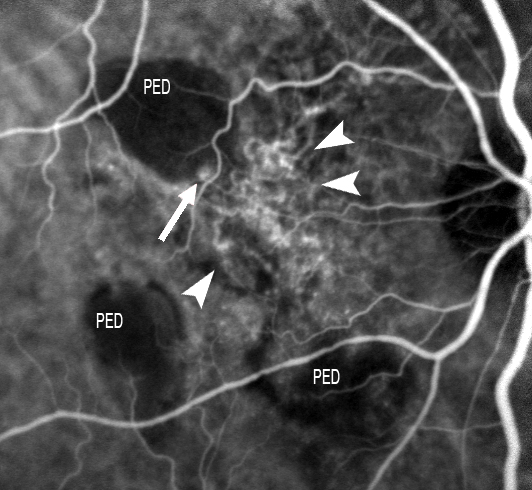
Figure 17. ICGA is able to identify two elements in these anomalies: polypoidal structures projecting from the internal choroid towards the retina, and a BVN manifesting with early vascular hyperfluorescence. The figure shows this choroidal vascular network (short white arrows), with the formation of a polyp at marginal level (large white arrow).
The polyps are difficult to identify on FA, as they are located beneath the RPE. On ICGA, however, the polyps are clearly visible as single or multiple vascular aneurysms with a diameter of 100-500 μm which fill after a short delay and remain hyperfluorescent until the late phases. These lesions are often located in the juxtapapillary zone, but can also be found at macular level, in the vascular arches, and even at peripheral level. The choroidal vessels in the area of the polyps may appear irregular and dilated, forming a fine vascular network. The angiographic sequence would be as follows: filling of the fine choroidal vessels and polyps with early choroidal vascular hyperfluorescence when the dye flows through the network of anomalous vessels. This fluorescence is maintained over time and disappears as the dye washes out in the late phases, in the presence of polypoidal lesions without active leakage (Figure 18).
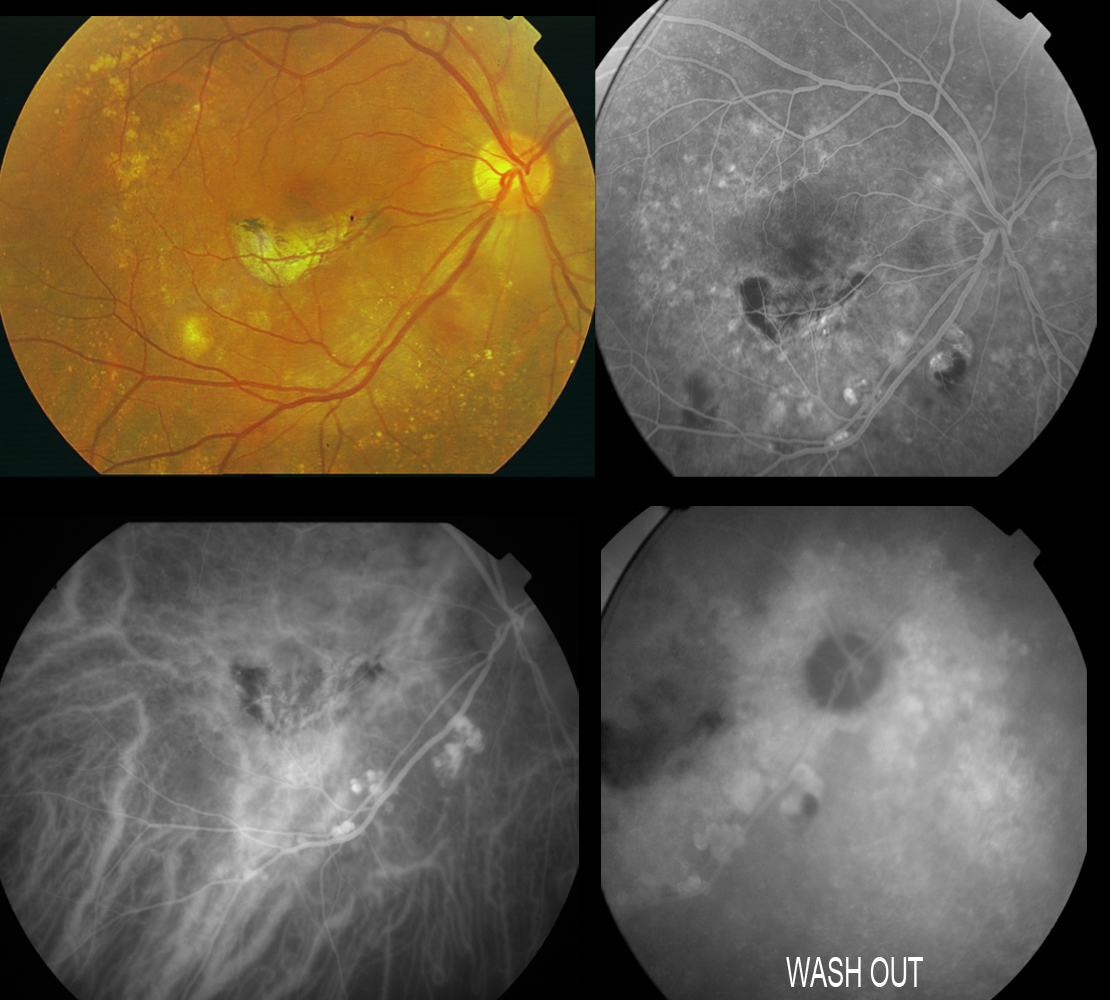
Figure 18. Patient diagnosed with IPCV subjected to laser treatment in the macular area. Scantly active polyps persist at inferior temporal arcade level, evidencing dye washout in the last phases of the angiogram. The angiographic sequence would be as follows: filling of the fine choroidal vessels and polyps with early choroidal vascular hyperfluorescence when the dye flows through the network of anomalous vessels. This fluorescence is maintained over time and disappears as the dye washes out in the late phases, in the presence of polypoidal lesions without active leakage.
Guidelines have recently been published for the clinical diagnosis and treatment of IPCV(139), in which ICGA is regarded as the gold standard for the diagnosis of this disorder. This panel of experts considers that such angiography should be performed when routine ophthalmoscopic examination indicates a serosanguineous maculopathy with one of the following features:
- Red-orange subretinal nodules visible with the ophthalmoscope.
- Spontaneous massive subretinal hemorrhage.
- Notched or hemorrhagic PED.
- Occlusion of polyps with lowresponse rate to anti-VEGF therapy.
In conclusion, ICGA is the gold standard for diagnosis of IPCV, which is defined as the presence of single or multiple focal nodular areas of hyperfluorescence arising from the choroidal circulation within the first 6 minutes after injection of ICG, with or without an associated BVN(139).
These authors propose algorithms for both the diagnosis and the treatment of the disease. IPCV may be classified clinically as: quiescent (polyps in the absence of subretinal or intraretinal fluid or haemorrhage), exudative (exudation without haemorrhage, which includes variously sensory retinal thickening, neurosensory detachment, PED, and subretinal lipid exudation) or hemorrhagic (any subretinal or sub-RPR haemorrhage with or without other exudative characteristics). Treatment should be initiated for active and symptomatic IPCV, and can be considered for active asymptomatic presentations (Figure 19).
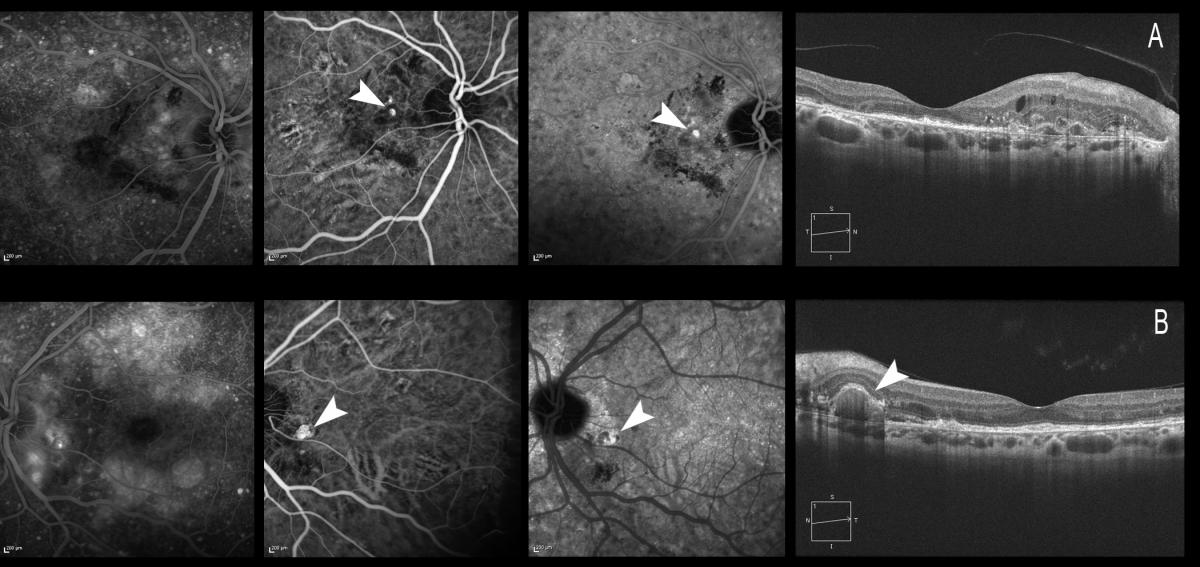
Figure 19. Treatment should be initiated for active and symptomatic IPCV, and can be considered for active asymptomatic presentations. The figure shows both eyes of the same patient. (A) The patient is symptomatic, with a visual acuity of 0.6 in the right eye. The polypoidal lesions can be observed with ICGA, and retinal thickening and intraretinal cysts can be seen by OCT. (B) The patient maintains a visual acuity of 1.0 in the left eye. ICGA shows a peripapillary polyp, likewise visible in the OCT acquisition, but there is no retinal edema, and the patient therefore remains asymptomatic.
ICGA is necessary not only for the diagnosis but also for continuous monitoring of the disease, which may present polyp relapse or the appearance of new polyps from the BVN, with phases of quiescence, exudation or hemorrhage (Figure 20).
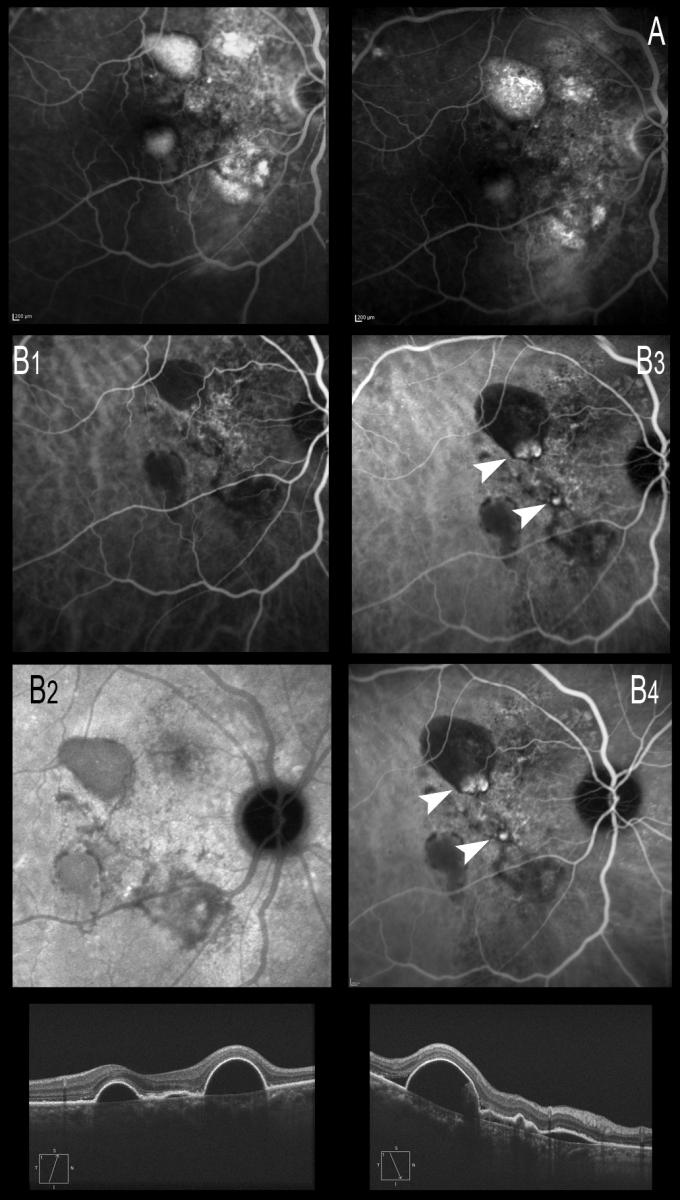
Figure 20. ICGA is necessary not only for the diagnosis but also for continuous monitoring of the disease, which may present polyp relapse or the appearance of new polyps from the BVN, with phases of quiescence, exudation or hemorrhage. (A) Late phases of FA showing the hyperfluorescent PEDs. (B) ICGA image. (B1 and B2) Only the PEDs are seen, and the BVN alteration is noted in early phases with ICGA. (B3 and B4) Formation of polyps from the BVN, visible with ICGA 6 months later.
Focal photocoagulation with ICGA-guided thermal laser still plays a role in the treatment of extrafoveal polyps, but it is not recommended in application to juxta- or subfoveal lesions(139,140). In these cases, PDT has been the treatment of choice, with maintenance or improvement of patient visual acuity in 80-95% of the cases(141-146) (Figure 21).
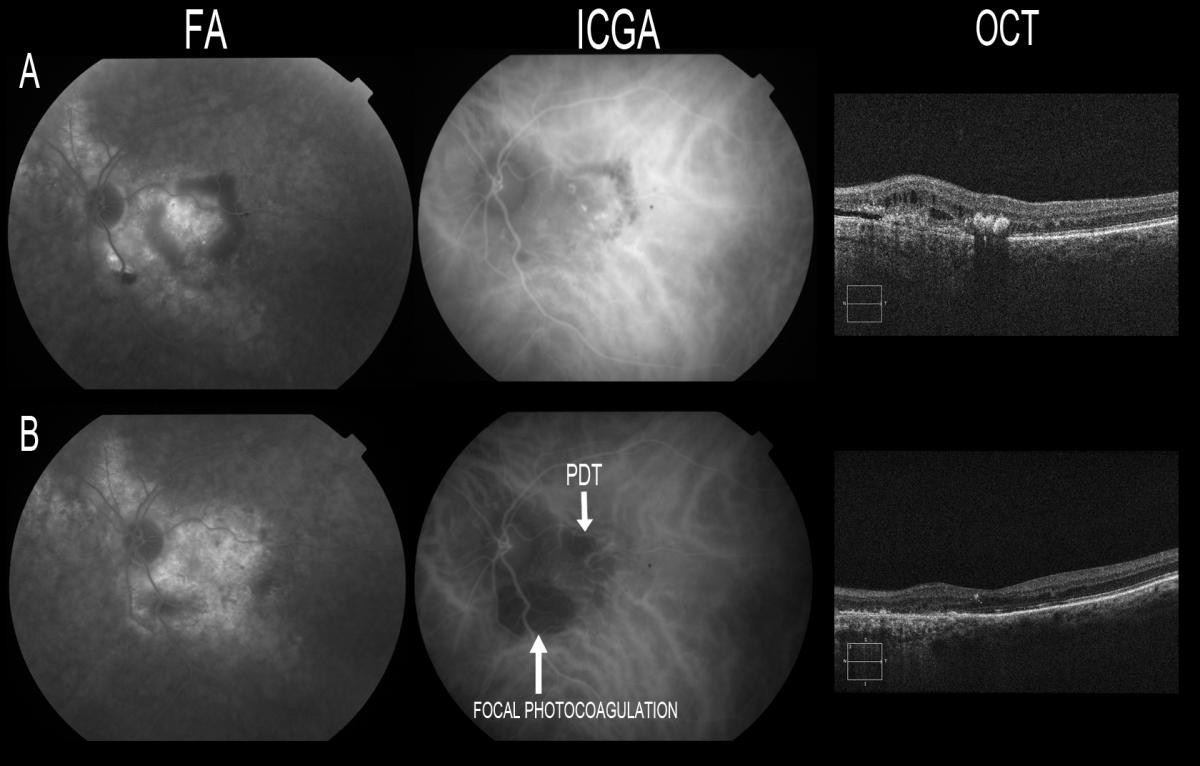
Figure 21. Focal photocoagulation with ICGA-guided thermal laser still plays a role in the treatment of extrafoveal polyps, but it is not recommended in application to juxta- or subfoveal lesions. (B) Resolution of the polyps with atrophy scarring secondary to thermal laser in extrafoveal polyps at inferior temporal arcade level and after PDT at macular level. OCT shows resolution of the retinal thickening, with disappearance of the hard exudate.
However, long-term follow-up studies have described frequent recurrences after PDT(142, 147). In some cases PDT is unable to fully occlude the polyps, though in other cases persistence of the BVN can give rise to new active polypoidal lesions. As a result, periodic controls with ICGA are needed during follow-up to determine whether there are persistent or recurrent lesions with polyps or BVN.
Although recurrence and retreatments do not seem to decrease best corrected visual acuity, long-term studies are still needed in order to evaluate the efficacy and safety of PDT treatment of IPVC(142). PDT can modified the natural progression of the polypoidal CNV as a result of ischemia and increased VEGF expression(148).
After the introduction of anti-VEGF therapy, different studies reported the efficacy of combined treatment with PDT and anti-VEGF agents for IPCV(149,150), as well as the way to minimize the complications associated with the use of PDT, and the need to use selective(151) or with reduced fluence PDT(152).
The Everest study(153) concluded that V-PDT combined with ranibizumab 0.5 mg or alone was superior to ranibizumab monotherapy. In our own experience with long-term patient follow-up(154), the association of ICGA-guided PDT for the occlusive therapy of polyps and intravitreous ranibizumab is effective in prolonging the recurrence-free interval, as well as in maintaining good visual acuity (Figure 22).
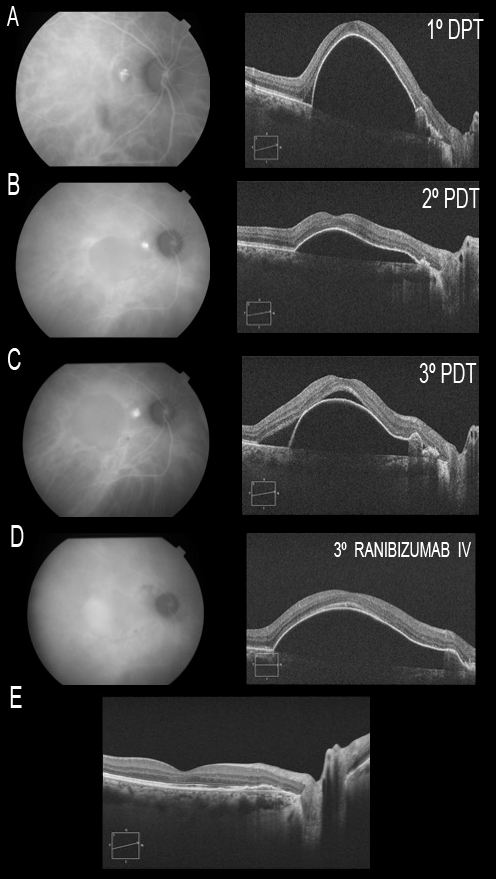
Figure 22. The association of ICGA-guided PDT for the occlusive therapy of polyps and intravitreous ranibizumab is effective in prolonging the recurrence-free interval, as well as in maintaining good visual acuity. Patient with a single juxtapapillary polyp associated to a large PED at macular level. Three PDT sessions are performed (A, B and C), without resolution of the condition. Indeed, worsening is noted, with the appearance of neurosensory detachment of the fovea (C). At the time where the polyp is not visualized by ICGA, and since the PED persists, three intravitreous injections of ranibizumab are administered, followed by resolution of the condition (E). The patient remained stable, with a visual acuity of 20/20 until month 36 of follow up.
Although the efficacy and safety of PDT and ranibizumab versus PDT and bevacizumab remain to be established, the experts have concluded(23) that PDT with standard fluence guided by ICGA with or without the combination of three intravitreous injections of ranibizumab 0.5 mg on a monthly basis is the most widely recommended treatment for this disease. ICGA-guided thermal laser photocoagulation can be considered for extrafoveal polyps.
The use of ICGA is very important for guiding laser treatment and PDT, both as regards the indication of such treatments and to minimize the spot size. This in turn contributes to limit as far as possible the risk of atrophy and other side effects derived from the multiple treatments usually required in the long-term follow-up of these patients.
A study with ICGA may in some cases help to unmask patients purportedly refractory to anti-VEGF therapy(155), because it allows a precise diagnosis and adequate treatment indication.
ICGA will retain a key role in the management of PCV because of its ability to better identify polyps although SS-OCTA is effective at detecting vascular network(156). However, SS-OCTA may be more sensitive than SD-OCT in detecting early recurrence.
On the other hand, the combination of En Face and cross-sectional OCTA images provides anatomical information about polypoidal structures that is comparable to ICGA. OCTA is safe and can provide supplementary information but due to its limitations it does not replace ICGA in a clinical setting nowadays(157-159).
References - Diagnostic usefulness of indocyanine green angiography (ICGA) in age-related macular degeneration (AMD)
References - Diagnostic usefulness of indocyanine green angiography (ICGA) in age-related macular degeneration (AMD)
- Congdon NG, Friedman DS, Lietman T. Important Causes of Visual Impairment in the World Today. JAMA. 2003;290(15):2057-2060.
- Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564-572.
- Bressler NM. Age-Related Macular Degeneration Is the Leading Cause of Blindness. JAMA. 2004;291(15):1900-1901.
- Alfredo Pece, Claudio Azzolini, Maurizio Battaglia Parodi, Ferdinando Bottoni, Paola Danzi, Simone Donati, Ugo Introini, Vincenzo Pucci, Francesco Semeraro, and Francesco Viola. Consensus on the diagnosis, treatment and follow-up of patients with age-related macular degeneration eligible for ranibizumab. Expert Review of Ophthalmology. 2012;7(3):219-225.
- Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68(8):1029–36.
- Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012;87(1):77–88.
- Waisbourd M, Loewenstein A, Goldstein M, Leibovitch I. Targeting vascular endothelial growth factor: a promising strategy for treating age-related macular degeneration. Drugs Aging. 2007; 24(8):643–62.
- Agarwal A, Rhoades WR, Hanout M, Soliman MK, Sarwar S, Sadiq MA, Sepah YJ, Do DV, Nguyen QD. Management of neovascular age related macular degeneration: current state-of-the art care optimizing visual outocomes and therapies in development. Clini Ophtahalmol. 2015 Jun;9:1001-15.
- Shoughy SS, Kozak I. Selective and complementary use of Optical Coherence Tomography and Fluorescein Angiography in retinal practice. Eye Vis (Lond). 2016;17;3:26.
- Hope-Ross M, Yannuzzi LA, Gragoudas ES, TFyer DR, Slakter JS, Sorenson JA, Krupsky S, Orlock DA, Puliafito CA. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101:529-533.
- Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960 Apr;39:592-600.
- Baker KJ. Binding of sulfobromophthalein (BSP) sodium and indocyanine green (ICG) by plasma alpha-1 lipoproteins. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):957-63.
- Brown N, Strong R. Infrared fundus angiography. Br J Ophthalmol. 1973 Oct;57(10):797-802.
- Fineman MS, Maguire JI, Fineman SW, Benson WE. Safety of indocyanine green angiography during pregnancy. Arch Ophthalmol. 2001; 119:353-355.
- Guyer DR and Yannuzzi LA. Occult choroidal neovascularization. In Flower RW. Indocyanine green angiography. Yannuzzi LA, Flower RW, Slakter JS. Ed. Mosby. St. Louis, Missouri. 199:157-180.
- Guyer DR, Puliafito CA, Mones JM, Friedman E, Chang W, Verdooner SR. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology. 1992 Feb;99(2):287-91.
- Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12(3):191-223.
- Kogure K, David NJ, Yamanouchi U, Choromokos E. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol. 1970 Feb;83(2):209-14.
- Flower RW. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Invest Ophthalmol. 1973; 12:881-895.
- Desmettre T, Devoisselle JM, Mordon S. Fluorescence properties and metabolic features of indocyanine green as related to angiography. Surv Ophthalmol. 2000; 45:15-27.
- Obana A, Miki T, Hayashi K, Takeda M, Kawamura A, Mutoh T, Harino S, Fukushima I, Komatsu H, Takaku Y. Survey of complications of indocyanine green angiography in Japan. Am J Ophthalmol. 1994;118:749-753.
- Cohen SY, Dubois L, Quentel G, Gaudric A. Is indocyanine green angiography still relevant? Retina. 2011 Feb;31(2):209-21.
- Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12:191–223.
- Hayashi K, Hasegawa Y, Tokoro T, De Laey JJ. Value of indocyanine green angiography in the diagnosis of occult choroidal neovascular membrane. Jpn J Clin Ophthalmol. 1988;42:827–829.
- Hayashi K, Hasegawa Y, Tokoro T, De Laey JJ. Clinical application of indocyanine green angiography to occult choroidal neovascularisation. Jpn J Clin Ophthalmol. 1989;43:57–65.
- Sorenson JA, Yannuzzi LA, Slakter JS, Guyer DR, Ho AC, Orlock DA. A pilot study of digital indocyanine green videoangiography for recurrent occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1994;112:473–479.
- Slakter JS, Yannuzzi LA, Sorenson JA, Guyer DR, Ho AC, Orlock DA. A pilot study of indocyanine green videoangiography-guided laser photocoagulation of occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1994;112:465–472.
- Yannuzzi LA, Negrao S, Iida T et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001;21:416–434.
- Kuhn D, Meunier I, Soubrane G, Coscas G. Imaging of chorioretinal anastomoses in vascularized retinal pigment epithelium detachments. Arch Ophthalmol. 1995;113:1392–1398.
- Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D, Matsumoto Y, Sorenson JA, Yannuzzi L. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina. 2008;28:201–211.
- Shiraga F, Ojima Y, Matsuo T, Takasu I, Matsuo N. Feeder vessel photocoagulation of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 1998;105:662–669.
- Flower RW. Experimental studies of indocyanine green dye-enhanced photocoagulation of choroidal neovascularization feeder vessels. Am J Ophthalmol. 2000;129:501–512.
- Desatnik H, Treister G, Alhalel A, Krupsky S, Moisseiev J. ICGA-guided laser photocoagulation of feeder vessels of choroidal neovascular membranes in age-related macular degeneration. Indocyanine green angiography. Retina 2000; 20:143–150.
- Lafaut BA, Bartz-Schmidt KU, Vanden Broecke C, Aisenbrey S, De Laey JJ, Heimann K. Clinicopathological correlation in exudative age related macular degeneration: histological differentiation between classic and occult choroidal neovascularisation. Br J Ophthalmol. 2000;84:239–243.
- Bressler NM; Treatment of Age-Related Macular Degener- ation with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol. 2001;119:198–207.
- Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age- related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131:541–560.
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431.
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age related macular degeneration. N Engl J Med. 2006;355:1432–1444.
- Arias L. Treatment of retinal pigment epithelial detachment with antiangiogenic therapy. Clin Ophthalmol. 2010;4:369–374.
- Gass JD, Agarwal A, Lavina AM, Tawansy KA. Focal inner retinal hemorrhages in patients with drusen: an early sign ofoccult choroidal neovascularization and chorioretinal anastomosis. Retina. 2003;23:741–751.
- Matsumoto H, Sato T, Kishi S. Tomographic features of intraretinal neovascularization in retinal angiomatous proliferation. Retina 2010;30:425–430.
- Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147:644–652.
- Keane PA, Sadda SR. Imaging chorioretinal vascular disease. Eye (Lond). 2010;24:422–427.
- Arnold JJ, Quaranta M, Soubrane G, Sarks SH, Coscas G. Indocyanine green angiography of drusen. Am J Ophthalmol. 1997;124:344–356.
- Chang AA, Guyer DR, Orlock DR, Yannuzzi LA. Age-dependent variations in the drusen fluorescence on indocyanine green angiography. Clin Experiment Ophthalmol. 2003;31:300–304.
- Quaranta M, Buglione M, Lo Schiavo Elia R, Coscas G, Soubrane G. Indocyanine green angiography of basal laminar drusen in the retinal pigment epithelium associated with vitelliform macular degeneration. J Fr Ophtalmol. 1998;21:185–190.
- Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15:183–191.
- Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148:733–743.
- Giani A, Pellegrini M, Carini E, Peroglio Deiro A, Bottoni F, Staurenghi G. The dark atrophy with indocyanine green angiography in Stargardt disease. Invest Ophthalmol Vis Sci. 2012 Jun 26;53(7):3999-4004.
- Guyer DR, Yannuzzi LA, Slakter JS, et al. Classification of choroidal neovascularizaton by digital indocyanine green videoangiography. Ophthalmology. 1996;103:2054–60.
- Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003 Jan;110(1):15-21.
- Guyer DR, Yannuzzi LA, Ladas I, et al. Indocyanine green guided laser photocoagulation of focal spots at the edge of plaques of choroidal neovascularization. Arch Ophthalmol. 1996;114:693–7.
- Kim H, Lee SC, Kim SM, Lee JH, Koh HJ, Kim SS, Byeon SH, Kim M, Lee Cs. Identification of underlying causes of spontaneous submacular haemorrhage by indocyanine green angiography. Ophthalmologica. 2015;233(3-4):146-54.
- Hoang QV, Gallego-Pinazo R, Yannuzzi LA. Long-term follow-up of acute zonal occult outer retinopathy. Retina. 2013 Jul-Aug;33(7):1325-7.
- Sorenson JA, Yannuzzi LA, Slakter JS, Guyer DR, Ho AC, Orlock DA. A pilot study of digital indocyanine green videoangiography for recurrent occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1994 Apr;112(4):473-9.
- Coscas G. Chapter III: Age-Related Macular Degeneration. In: Atlas of indocyanine green angiography fluorescein angiography, ICG angiography and OCT correlations. Coscas G. pp 118. Ed. Elsevier September 2005. ISBN: 2-84299-729-8.
- Freund KB, Yannuzzi LA, Sorenson JA. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol. 1993 Jun;115(6):786-91.
- Haddad WM, Coscas G, Soubrane G. Eligibility for treatment and angiographic features at the early stage of exudative age related macular degeneration. Br J Ophthalmol. 2002 Jun;86(6):663-9.
- Gass JD. Serous retinal pigment epithelial detachment with a notch: a sign of occult choroidal neovascularization. 1984. Retina. 2003 Dec;23(6 Suppl):205-20.
- Yannuzzi LA, Hope-Ross M, Slakter JS, Guyer DR, Sorenson JA, Ho AC, Sperber DE, Freund KB, Orlock DA. Analysis of vascularized pigment epithelial detachments using indocyanine green videoangiography. Retina. 1994;14(2):99-113.
- Tan A, Simhaee D, Balaratnasingam C, Dansingani K, Yannuzzi L. A perspective on the nature and frequency of pigment epithelial detachment. Am J Ophthalmol. 2016 Dec;172:13-27.
- Yannuzzi LA, Negrao S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001; 21: 416-434.
- Parodi MB, Iacono P, Menchini F, Sheth S, Polini G, Pittino R, Bandello F. Intravitreal bevacizumab versus ranibizumab for the treatment of retinal angiomatous proliferation. Acta Ophthalmol. 2013 May;91(3):267-73.
- Yannuzzi LA. Degeneration: age-related macular degeneration. In: The Retinal Atlas. Philadelphia, PA: Saunders; 2010.
- Yannuzzi LA, Freund KB, Takahashi BS. Review of retinal angiomatous proliferation or type 3 neovascularization [editorial]. Retina. 2008;28:375–384.
- Massacesi AL, Sacchi L, Bergamini F, Bottoni F. The prevalence of retinal angiomatous proliferation in age-related macular degeneration with occult choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:89–92.
- Kuhn D, Meunier I, Soubrane G, Coscas G. Imaging of chorioretinal anastomoses in vascularised retinal pigment epithelial detachments. Arch Opthalmol. 1995,113:1392–1398.
- Querques G, Querques L, Forte R, Massamba N, Blanco R, Souied EH. Precursors of type 3 neovascularization: A Multimodal Imaging Analysis. Retina. 2013 Jun;33(6):1241-8.
- Hartnett ME, Weiter JJ, Staurenghi G, et al. Deep retinal vascular anomalous complexes in advanced age-related macular degeneration. Ophthalmology. 1996;103:2042–2053.
- Bottoni F, Massacesi A, Cigada M, et al. Treatment of retinal angiomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferation. Arch Ophthalmol. 2005;123: 1644–1650.
- BresslerNM. Retinal anastomosis to choroidal neovascularization: a bum rap for a difficult disease. Arch Ophthalmol. 2005;123:1741–1743.
- Ghazi NG, Knape RM, Kirk TQ, Tiedeman JS, Conway BP. Intravitreal bevacizumab (avastin) treatment of retinal angiomatous proliferation. Retina. 2008 May;28(5):689-95.
- Axel-Siegel R, Bourla D, Priel E, Yassur Y, Weinberger D. Angiographic and flow patterns of retinal choroidal anastomoses in age-related macular degeneration with occult choroidal neovascularization. Ophthalmology. 2002 Sep;109(9):1726-36.
- Gross NE, Aizman A, Brucker A, Klancnik JM Jr, Yannuzzi LA. Nature and risk of neovascularization in the fellow eye of patients with unilateral retinal angiomatous proliferation. Retina. 2005;25(6):713–718.
- Campa C, Harding SP, Pearce IA, Beare NA, Briggs MC, Heimann H. Incidence of neovascularization in the fellow eye of patients with unilateral retinal angiomatous proliferation. Eye (Lond). 2010 Oct;24(10):1585-9.
- Koizumi H, Iida T, Saito M, Nagayama D, Maruko I. Choroidal circulatory disturbances associated with retinal angiomatous proliferation on indocyanine green angiography. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):515–520.
- McBain VA, Kumari R, Townend J, Lois N. Geographic atrophy in retinal angiomatous proliferation. Retina. 2011; 31(6):1043–1052.
- Kim JH, Kim JR, Kang SW, Kim SJ, Ha HS. Thinner Choroid and Greater Drusen Extent in Retinal Angiomatous Proliferation than in Typical Exudative Age-Related Macular Degeneration. J Ophthalmol. 2013;155:743–749.
- Mendis R, Leslie T, McBain VA, Lois N. Combined therapy for retinal angiomatous proliferation with intravitreal triamcinolone and argon laser photocoagulation. Br J Ophthalmol. 2008;92:1154–1156.
- Boscia F, Furino C, Sborgia L, Reibaldi M, Sborgia C. Photodynamic therapy for retinal angiomatous proliferation and pigment epithelium detachment. Am J Ophthalmol. 2004; 138:1077–1079.
- Boscia F, Parodi MB, Furino C, Reibaldi M, Sborgia G. Photodynamic therapy with verteporfin for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2006;244: 1224–1232.
- Kuroiwa S, Arai J, Gaun S, Lida T, Yoshimura N. Rapidly progressive scar formation after transpupillary thermotherapy in retinal angiomatous proliferation. Am J Ophthalmol. 2003;23:417–420.
- Hemeida TS, Keane PA, Dustin L, Sadda SR, Fauzi AA. Long- term visual and anatomic outcomes following anti-VEGF monotherapy for retinal angiomatous proliferation. Br J Ophthalmol. 2009;6:701–705.
- Gharbiya M, Allievi F, Recupero V, Martini D, Mazzeo L, Gabrieli CB. Intravitreal bevacizumab as primary treatment for retinal angiomatous proliferation: twelve-month results. Retina. 2009 Jun;29(6):740-9.
- Rouvas AA, Papakostas TD, Ladas ID. Ranibizumab for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2009 Dec;247(12):1719-20.
- Engelbert M, Zweifel SA, Freund KB. "Treat and extend" dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina. 2009 Nov-Dec;29(10):1424-31.
- Montero JA, Fernandez MI, Gomez-Ulla F, Ruiz-Moreno JM. Efficacy of intravitreal bevacizumab to treat retinal angiomatous proliferation stage II and III. Eur J Ophthalmol. 2009;19:448–451.
- Konstantinidis L, Mameletzi E, Mantel I, Pournovas JA, Zografos L, Ambresin A. Intravitreal ranibizumab (Lucentis) in the treatment of retinal angiomatous proliferation (RAP). Graefes Arch Clin Exp Ophthalmol. 2009;9:1165–1171.
- Meyerle CB, Freund KB, Iturralde D, et al. Intravitreal bevacizumab (Avastin) for retinal angiomatous proliferation. Retina. 2007;27:451–457.
- Hemeida TS, Keane PA, Dustin L, Sadda SR, Fawzi AA. Long-term visual and anatomical outcomes following anti-VEGF monotherapy for retinal angiomatous proliferation. Br J Ophthalmol. 2010 Jun;94(6):701-5.
- Gupta B, Jyothi S, Sivaprasad S. Current treatment options for retinal angiomatous proliferans (RAP). Br J Ophthalmol. 2010 Jun;94(6):672-7.
- George S, Cooke C, Chakravarthy U. Exudative AMD subtypes and eligibility for treatment with ranibizumab. Eye (Lond). 2010 Jul;24(7):1247-51.
- Atmani K, Voigt M, Le Tien V, et al. Ranibizumab for retinal angiomatous proliferation in age-related macular degeneration. Eye. 2010;24:1193–1198.
- Querques G, Rousseau A, Forte R, et al. Longitudinal anatomical response of retinal-choroidal anastomosis to antivascular endothelial growth factor therapy. Retina. 2012;32:458–467.
- Hufendiek K, Hufendiek K, Panagakis G, Helbig H, Gamulescu MA. Visual and morphological outcomes of bevacizumab (Avastin®) versus ranibizumab (Lucentis®) treatment for retinal angiomatous proliferation. Int Ophthalmol. 2012 Jun;32(3):259-68.
- Kramann CA, Schöpfer K, Lorenz K, Zwiener I, Stoffelns BM, Pfeiffer N. Intravitreal ranibizumab treatment of retinal angiomatous proliferation. Acta Ophthalmol. 2012 Aug;90(5):487-91.
- Hsu TK, Liu JH, Lei J, Chao HM. Retinal angiomatous proliferation responds safely to a double dose (1.0 mg) of ranibizumab. Clin Exp Optom. 2013 Jan;96(1):112-6.
- Krebs I, Krepler K, Stolba U, Goll A, Binder S. Retinal angiomatous proliferation: combined therapy of intravitreal triamcinolone acetonide and PDT versus PDT alone. Graefes Arch Clin Exp Ophthalmol. 2008 Feb;246(2):237-43.
- Bakri SJ, Ekdawi NS. Intravitreal triamcinolone and bevacizumab combination therapy for refractory choroidal neovascularization with retinal angiomatous proliferation.Eye (Lond). 2008 Jul;22(7):978-80.
- Saito M, Shiragami C, Shiraga F, Nagayama D, Iida T. Combined intravitreal bevacizumab and photodynamic therapy for retinal angiomatous proliferation. Am J Ophthalmol. 2008 Dec;146(6):935-41.e1.
- Montero JA, Ruiz-Moreno JM, Sanabria MR, Fernandez-Munoz M. Efficacy of intravitreal and periocular triamcinolone associated with photodynamic therapy for treatment of retinal angiomatous proliferation. Br J Ophthalmol. 2009 Feb;93(2):166-70.
- Rouvas AA, Papakostas TD, Vavvas D, Vergados I, Moschos MM, Kotsolis A, Ladas ID. Intravitreal ranibizumab, intravitreal ranibizumab with PDT, and intravitreal triamcinolone with PDT for the treatment of retinal angiomatous proliferation: a prospective study. Retina. 2009 Apr;29(4):536-44.
- Shima C, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Tano Y. One-year results of combined photodynamic therapy and intravitreal bevacizumab injection for retinal pigment epithelial detachment secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009 Jul;247(7):899-906.
- Lo Giudice G, Gismondi M, De Belvis V, Cian R, Tavolato M, Galan A. Single-session photodynamic therapy combined with intravitreal bevacizumab for retinal angiomatous proliferation. Retina. 2009 Jul-Aug;29(7):949-55.
- Saito M, Shiragami C, Shiraga F, Kano M, Iida T. Comparison of intravitreal triamcinolone acetonide with photodynamic therapy and intravitreal bevacizumab with photodynamic therapy for retinal angiomatous proliferation. Am J Ophthalmol. 2010 Mar;149(3):472-81.
- Sahu AK, Narayanan R. Intravitreal ranibizumab, intravitreal ranibizumab with photodynamic therapy (PDT), and intravitreal triamcinolone with PDT for the treatment of retinal angiomatous proliferation. Retina. 2010 Jun;30(6):981.
- Viola F, Mapelli C, Villani E, Tresca Carducci F, Vezzola D, Ratiglia R. Sequential combined treatment with intravitreal bevacizumab and photodynamic therapy for retinal angiomatous proliferation. Eye (Lond). 2010 Aug;24(8):1344-51.
- Lee MY, Kim KS, Lee WK. Combination therapy of ranibizumab and photodynamic therapy for retinal angiomatous proliferation with serous pigment epithelial detachment in Korean patients: twelve-month results. Retina. 2011 Jan;31(1):65-73.
- Nakano S, Honda S, Oh H, Kita M, Negi A. Effect of photodynamic therapy (PDT), posterior subtenon injection of triamcinolone acetonide with PDT, and intravitreal injection of ranibizumab with PDT for retinal angiomatous proliferation. Clin Ophthalmol. 2012;6:277-82.
- Saito M, Iida T, Kano M. Combined intravitreal ranibizumab and photodynamic therapy for retinal angiomatous proliferation. Am J Ophthalmol. 2012 Mar;153(3):504-514.
- Lee MY, Kim KS, Lee WK. Combined intravitreal ranibizumab and photodynamic therapy for retinal angiomatous proliferation. Am J Ophthalmol. 2012 May;153(5):1004-5; author reply 1005.
- Rouvas AA, Chatziralli IP, Theodossiadis PG, Moschos MM, Kotsolis AI, Ladas ID. Long-term results of intravitreal ranibizumab, intravitreal ranibizumab with photodynamic therapy, and intravitreal triamcinolone with photodynamic therapy for the treatment of retinal angiomatous proliferation. Retina. 2012 Jun;32(6):1181-9.
- Shirakata Y, Shiragami C, Yamashita A, Nitta E, Fujiwara A, Shiraga F. One-year results of bevacizumab intravitreal and posterior sub-Tenon injection of triamcinolone acetonide with reduced laser fluence photodynamic therapy for retinal angiomatous proliferation. Jpn J Ophthalmol. 2012 Nov;56(6):599-607.
- Saito M, Iida T, Kano M. Two-year results of combined intravitreal anti-VEGF agents and photodynamic therapy for retinal angiomatous proliferation. Jpn J Ophthalmol. 2013 Mar;57(2):211-20.
- Forooghian F, Cukras C, Chew EY. Retinal angiomatous proliferation complicated by pigment epithelial tear following intravitreal bevacizumab treatment.Can J Ophthalmol. 2008 Apr;43(2):246-8.
- Gutfleisch M, Heimes B, Schumacher M, Dietzel M, Lommatzsch A, Bird A, Pauleikhoff D. Long-term visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigment epithelial detachment in AMD. Eye (Lond). 2011 Sep;25(9):1181-6.
- Introini U, Torres Gimeno A, Scotti F, Setaccioli M, Giatsidis S, Bandello F. Vascularized retinal pigment epithelial detachment in age-related macular degeneration: treatment and RPE tear incidence. Graefes Arch Clin Exp Ophthalmol. 2012 Sep;250(9):1283-92.
- Reche-Frutos J, Calvo-Gonzalez C, Pérez-Trigo S, Fernandez-Perez C, Donate-Lopez J, Garcia-Feijoo J. Ranibizumab in retinal angiomatous proliferation (RAP): influence of RAP stage on visual outcome. Eur J Ophthalmol. 2011 Nov-Dec;21(6):783-8.
- Kim JH, Chang YS, Kim JW, Lee TG, Kim H. Diagnosis of type 3 neovascularization based on optical coherence tomography images. Retina 2016;36: 1506-1515.
- Yannuzzi LA. Idiopathic polypoidal choroidal vasculopathy. Macula Society Meeting, 1982, Miami, FL.
- Kleiner RC, Brucker AJ, Johnston RL. The posterior uveal bleeding syndrome. Retina. 1990;10:9–17.
- Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10: 1–8.
- Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:4729–4737.
- Stern RM, Zakov ZN, Zegarra H, Gutman FA. Multiple recurrent serosanguineous retinal pigment epithelial detachments in black women. Am J Ophthalmol. 1985;100:560–569.
- Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997;115:478–485.
- Chang YC, Wu WC. Polypoidal choroidal vasculopathy in Taiwanese patients. Ophthalmic Surg Lasers Imaging. 2009; 40:576–58.
- Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004;49:25–37.
- Lafaut BA, Leys AM, Snyers B, et al. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238:752–759.
- Yannuzzi LA; Ciardella A, Spaide RF, Rabb M, Orlock DA. The expanding clinical spectrum of idiopatic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997; 115: 478-85.
- Spaide RF, Yannuzzi LA, Slakter JS; Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopatic polypoidal choroidal vasculopathy. Retina. 1995; 15: 100-110.
- Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy.Br J Ophthalmol. 2005; 89: 602-607.
- MacCumber MW, Dastgheib K, Bressler NM, Chan CC, Harris M, Fine S et al. Clinicopathologic correlation of the multiple recurrent serosanguineous retinal pigment epithelial detachments syndrome. Retina. 1994; 14: 143-152.
- Lafaut BA, Aisenbrey S, Van den Broecke C, Bartz-Schmidt KU, Heimann K. Polypoidal choroidal vasculopathy pattern in age-related macular degeneration. A clinicopathologic correlation. Retina. 2000; 20: 650-54.
- Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002; 86:321-27.
- Rosa RH Jr, Davis JL, Eifrig CW, Clinicopathologic correlation of idiopatic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002; 120: 502-508.
- Kuriwa S, Tateiwa H, Hisatomi T, Ishibashi T, Yoshimura N. Pathologic features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Exp Ophthalmol. 2004;32:292-302.
- Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol 2002;86:1093-98.
- Nakajima M, Yuzawa M, Shimada H, Mori R. Correlation between indocyanine green angiographic findings and histopathology of polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2004; 48: 249-55.
- Koh AH, Chen LJ, Chen SJ, Chen Y, Giridhar A, Iida T, Kim H, Yuk Yau Lai T, Lee WK, Li X, Han Lim T, Ruamviboonsuk P, Sharma T, Tang S, Yuzawa M; on behalf of the Expert PCV Panel. Polypoidal Choroidal Vasculopathy: Evidence-based Guidelines for Clinical Diagnosis and Treatment. Retina. 2013 Apr;33(4):686-716.
- Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye (Lond). 2009;23:145–148.
- Spaide RF, Donsoff I, Lam DL, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002; 22(5):529–535.
- E. Akaza, M. Yuzawa, Y. Matsumoto, S. Kashiwakura, K. Fujita, and R. Mori, “Role of photodynamic therapy in polypoidal choroidal vasculopathy,” Jpn J Ophthalmol. 2007;51(4):270–277.
- Gomi F, Ohji M, Sayanagi K, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115(1):141–146.
- Akaza E, Mori R, Yuzawa M. Long-term results of photodynamic therapy of polypoidal choroidal vasculopathy. Retina. 2008;28(5):717–722.
- Otani A, Sasahara M, Yodoi Y, et al. Indocyanine green angiography: guided photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007;144(1):7–e1.
- Silva RM, Figueira L, Cachulo ML, Duarte L, Faria de Abreu JR, Cunha-Vaz JG. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2005 Oct;243(10):973-9.
- Kurashige Y, Otani A, Sasahara M, et al. Two-year results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2008;146(4):513–22.
- Akaza E, Yuzawa M, Mori R. Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2011 Jan;55(1):39-44.
- Kim SJ, Yu HG. Efficacy of combined photodynamic therapy and intravitreal bevacizumab injection versus photo- dynamic therapy alone in polypoidal choroidal vasculopathy. Retina. 2011; Oct;31(9):1827-34.
- Lai TY, Chan WM, Liu DT, Luk FO, Lam DS. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy.Br J Ophthalmol. 2008 May;92(5):661-6.
- Eandi CM, Ober MD, Freund KB, Slakter JS, Yannuzzi LA. Selective photodynamic therapy for neovascular age-related macular degeneration with polypoidal choroidal neovascularization. Retina. 2007 Sep;27(7):825-31.
- Ricci F, Calabrese A, Regine F, Missiroli F, Ciardella AP. Combined reduced fluence photodynamic therapy and intravitreal ranibizumab for polypoidal choroidal vasculopathy. Retina. 2012 Jul;32(7):1280-1288.
- Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012 Sep;32(8):1453-64.
- Fernández M, Gil M, Gomez-Ulla F, Charlón P. Verteporfin photodynamic therapy combined with intravitreal ranibizumab for polypoidal choroidal vasculopathy controversy concerning long-term followup. Case Rep Med. 2012;2012:897097.
- Stangos AN, Gandhi JS, Nair-Sahni J, Heimann H, Pournaras CJ, Harding SP Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol. 2010 Nov;150(5):666-73.
- Cheung CM, Yanagi Y, Mohla A, Lee SY, Mathur R, Chan CM, Yeo I, Wong TY. Characterization and differentiation of polypoidal choroidal vasculopathy using swept source optical coherence tomography angiography. Retina. 2016 Nov 8. 0:1–11 [Epub ahead of print].
- Novais EA, Ferrara D, Waheed NK. Optical coherence tomography in polypoidal choroidal vasculopathy disease. Clin Exp Ophthalmol. 2015 Dec;43(9):779-81.
- Inoue M, Balaratnasingam C, Freund KB. Optical coherence tomography angiography of polypoidal choroidal vasculopathyand polypoidal choroidal neovascularization. Retina. 2015 Nov;35(11):2265-74.
- Kokame GT, Hirai K, Yanagihara R. Polypoidal Choroidal Vasculopathy: Imaging by Indocyanine Green Angiography and En Face Optical Coherence Tomography. JAMA Ophthalmol. 2015 Nov;133(11):e151886.